Question: - The gas phase zero order reaction A+2B - C is carried out in a CSTR. It was calculated that 45s space time is required
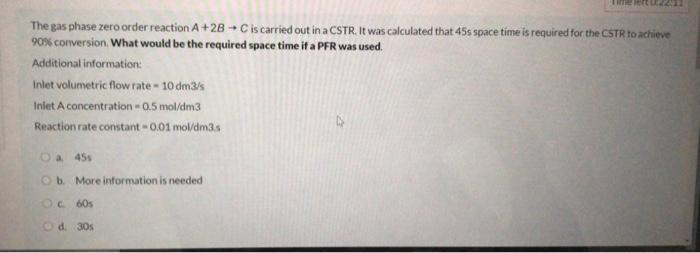

- The gas phase zero order reaction A+2B - C is carried out in a CSTR. It was calculated that 45s space time is required for the CSTR to achieve 90% conversion What would be the required space time if a PFR was used. Additional information: Inlet volumetric flow rate - 10 dm3/s Inlet A concentration - 0,5 mol/dm3 Reaction rate constant -0.01 mol/dm3.s a 455 Ob More information is needed 60s od 30s Ethylene is oxidized to produce ethylene-oxide in a Packed bed reactor. Calculate the initial Oxygen concentration if the total feed concentration is 100 kmol/m3 and the feed consists of 50% Ethylene and 50% Air O a. 40.5 kmol/m O b. 50.0 kmol/m OC 21.0 kmol/m Od 10.5 kmol/m2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


