Question: Consider a process that occurs at constant volume. The volume of a gas in a cylinder is 2.40L, the temperature is 30.0C and bofore the
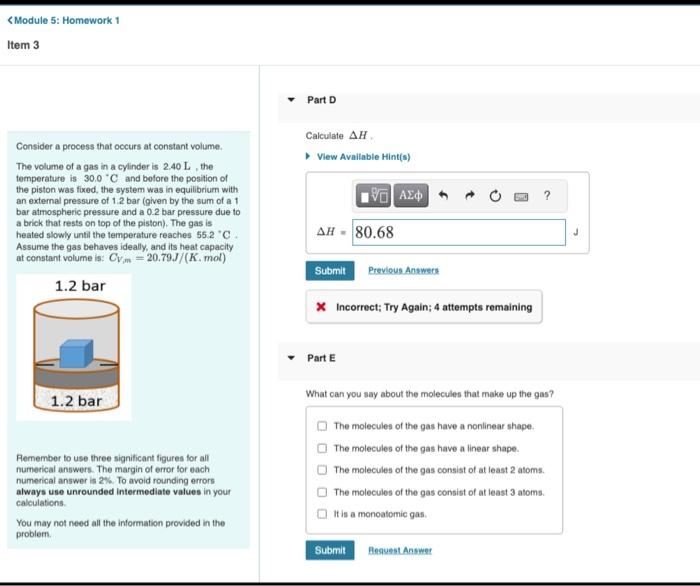

Consider a process that occurs at constant volume. The volume of a gas in a cylinder is 2.40L, the temperature is 30.0C and bofore the position of the piston was fixed, the system was in equilibrium with an external pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). The gas is heated slowly unti the temperature reaches 55.2C. H= Assume the gas behaves ideally, and its heat capacity at constant volume is: CV,m=20.79.J/(K,mol) X Incorrect; Try Again; 4 attempts remaining Part E What can you say about the molecules that make up the gas? The molecules of the gas have a nonlinear shape. Remember to use three significant figures for all numerical answers. The margin of error for each numerical answer is 2\%. To avoid rounding errors always use unrounded intermediate values in your calculations. The molecules of the gas have a linear shape. You may not need all the information provided in the problem. An insulated beaker with neglgible mass contains liguid water weh a mass of 0.240kg and a temperature of 67.7C How much ice at a terrperature of +162C must be dropped into the water so that the final hemperature of the system will be 35.0C ? Take the apecine heat of liquild water to be 4190J/kgK, the specific heat of ice to be 2100J/kgK, and the heat of fusion for waner to be 334kJ/kg. x incorrect: Try Again; 4 attempts remaining Estimate 1U22y. You can ignore the volume of liquids in this estimation and treat gases idealy Make sure so use conpatble units in your calculation. Express your anawer using two decimal places and include the appropriate units. x Incorrect; Try Again: 4 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


