Question: Consider a process that occurs at constant volume. What is the value of q ? (think about the sign first) The volume of a gas
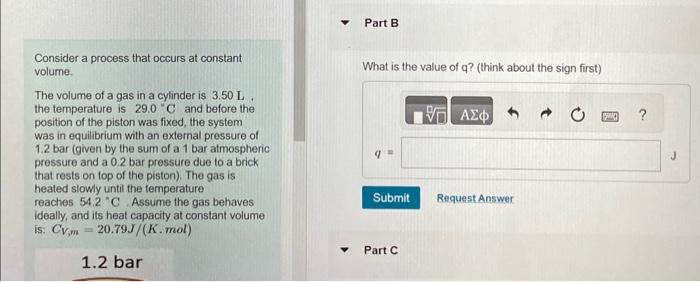
Consider a process that occurs at constant volume. What is the value of q ? (think about the sign first) The volume of a gas in a cylinder is 3.50L. the temperature is 29.0C and before the position of the piston was fixed, the system was in equilibrium with an extemal pressure of 1.2 bar (given by the sum of a 1 bar atmospheric pressure and a 0.2 bar pressure due to a brick that rests on top of the piston). The gas is heated slowly until the temperature reaches 54.2C. Assume the gas behaves ideally, and its heat capacity at constant volume is: CV,m=20.79J/(K.mol) Calculate U
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


