Question: consider ethanol as the compound to answer the question 5. Draw a phase change diagram (like the one below) labeling the temperatures at which the
consider ethanol as the compound to answer the question
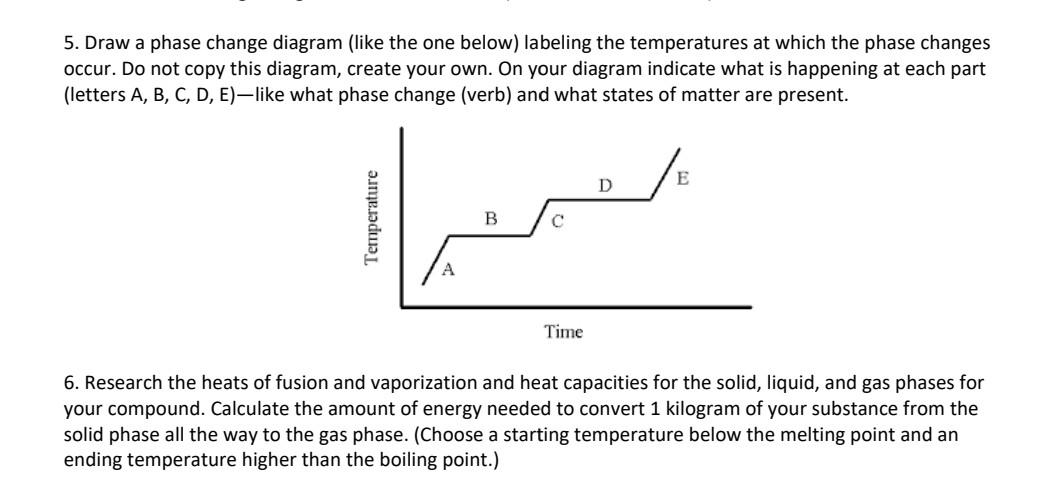
5. Draw a phase change diagram (like the one below) labeling the temperatures at which the phase changes occur. Do not copy this diagram, create your own. On your diagram indicate what is happening at each part (letters A, B, C, D, E)-like what phase change (verb) and what states of matter are present. 6. Research the heats of fusion and vaporization and heat capacities for the solid, liquid, and gas phases for your compound. Calculate the amount of energy needed to convert 1 kilogram of your substance from the solid phase all the way to the gas phase. (Choose a starting temperature below the melting point and an ending temperature higher than the boiling point.) 5. Draw a phase change diagram (like the one below) labeling the temperatures at which the phase changes occur. Do not copy this diagram, create your own. On your diagram indicate what is happening at each part (letters A, B, C, D, E)-like what phase change (verb) and what states of matter are present. 6. Research the heats of fusion and vaporization and heat capacities for the solid, liquid, and gas phases for your compound. Calculate the amount of energy needed to convert 1 kilogram of your substance from the solid phase all the way to the gas phase. (Choose a starting temperature below the melting point and an ending temperature higher than the boiling point.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


