Question: Consider oxygen in thermo-chemical equilibrium at 3,500 K. Plot the mole fractions of the mixture of gases present within a pressure range of 0.5atm and
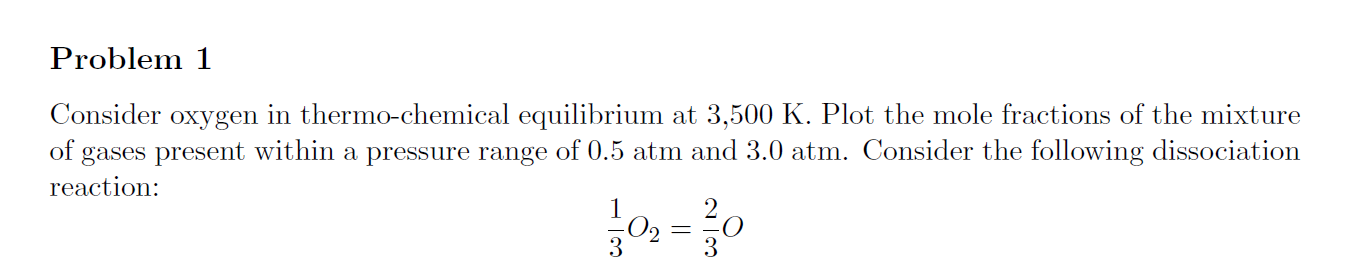
Consider oxygen in thermo-chemical equilibrium at 3,500 K. Plot the mole fractions of the mixture of gases present within a pressure range of 0.5atm and 3.0atm. Consider the following dissociation reaction: 31O2=32O
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


