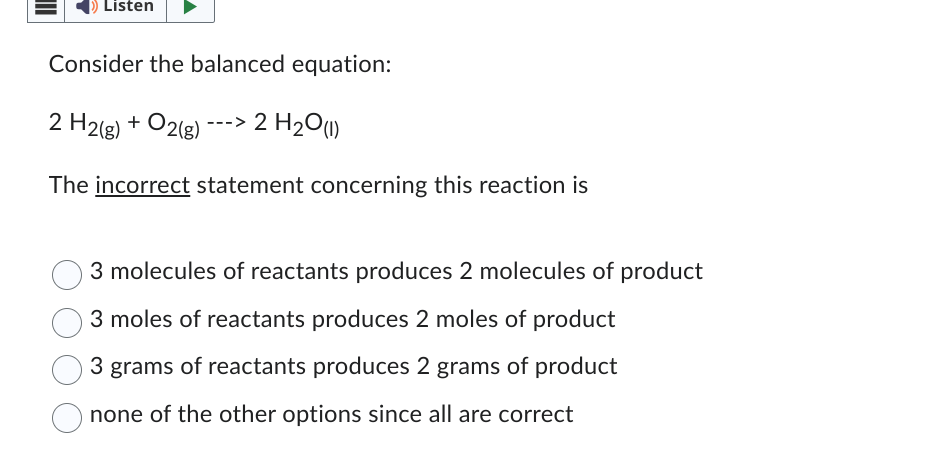
Question: Consider the balanced equation: 2 H 2 ( g ) + O 2 ( g ) - 2 H 2 O ( l ) The
Consider the balanced equation:
The incorrect statement concerning this reaction is
molecules of reactants produces molecules of product
moles of reactants produces moles of product
grams of reactants produces grams of product
none of the other options since all are correct
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


