Question: Consider the combustion reaction for octane (C8H18), which is a primary component of gasoline. 2C8H18+25O216CO2+18H2O How many moles of CO2 are emitted into the atmosphere
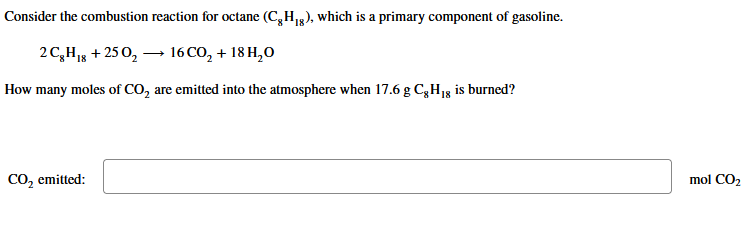
Consider the combustion reaction for octane (C8H18), which is a primary component of gasoline. 2C8H18+25O216CO2+18H2O How many moles of CO2 are emitted into the atmosphere when 17.6gC8H18 is burned
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


