Question: Consider the continuous process flow diagram below for the synthesis of methanol. CO/H CO/H2 Reactor Separator CH,OH CO H2 The raw materials are added in
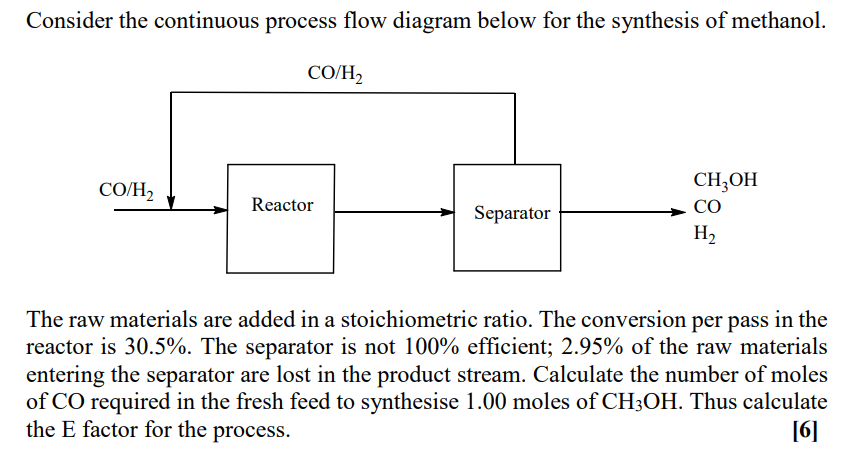
Consider the continuous process flow diagram below for the synthesis of methanol. CO/H CO/H2 Reactor Separator CH,OH CO H2 The raw materials are added in a stoichiometric ratio. The conversion per pass in the reactor is 30.5%. The separator is not 100% efficient; 2.95% of the raw materials entering the separator are lost in the product stream. Calculate the number of moles of CO required in the fresh feed to synthesise 1.00 moles of CH3OH. Thus calculate the E factor for the process. [6]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


