Question: Consider the cyclic process ABCA on a sample of 2.0 mol of an ideal gas as shown in figure (26-W4). The temperatures of the
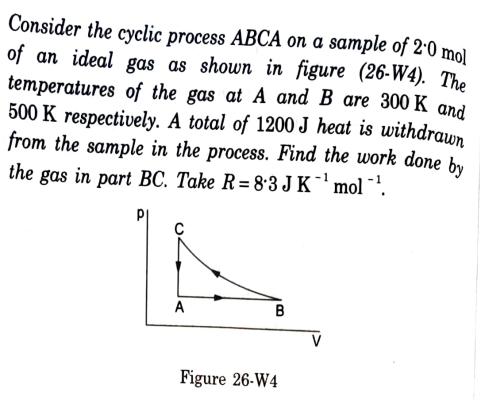
Consider the cyclic process ABCA on a sample of 2.0 mol of an ideal gas as shown in figure (26-W4). The temperatures of the gas at A and B are 300 K and 500 K respectively. A total of 1200 J heat is withdrawn from the sample in the process. Find the work done by the gas in part BC. Take R= 8.3 J K mol . A B Figure 26-W4
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


