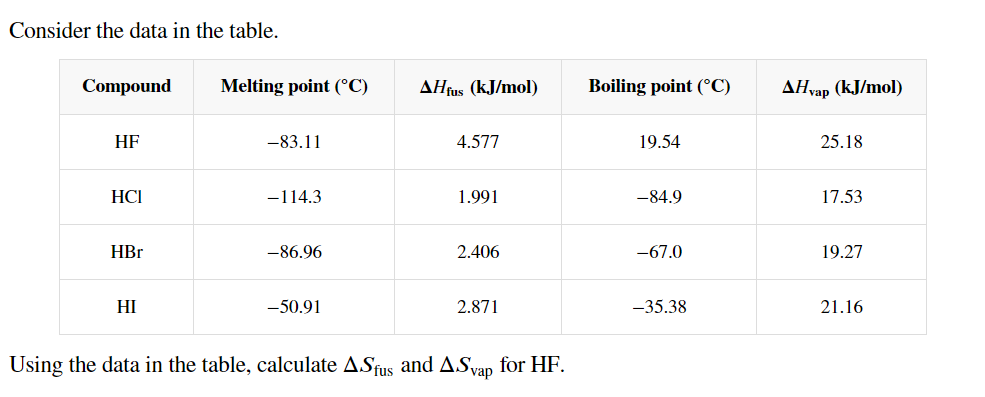
Question: Consider the data in the table. table [ [ Compound , Melting point ( C ) , H f u s ( k J
Consider the data in the table.
tableCompoundMelting point Boiling point Determine the entropy change when mol HF l freezes at atmospheric pressure.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


