Question: > Consider the diatomic molecules 02, 024, 07 and Om, where om corresponds to the neutral O2 molecule in its lowest excited state. Order the
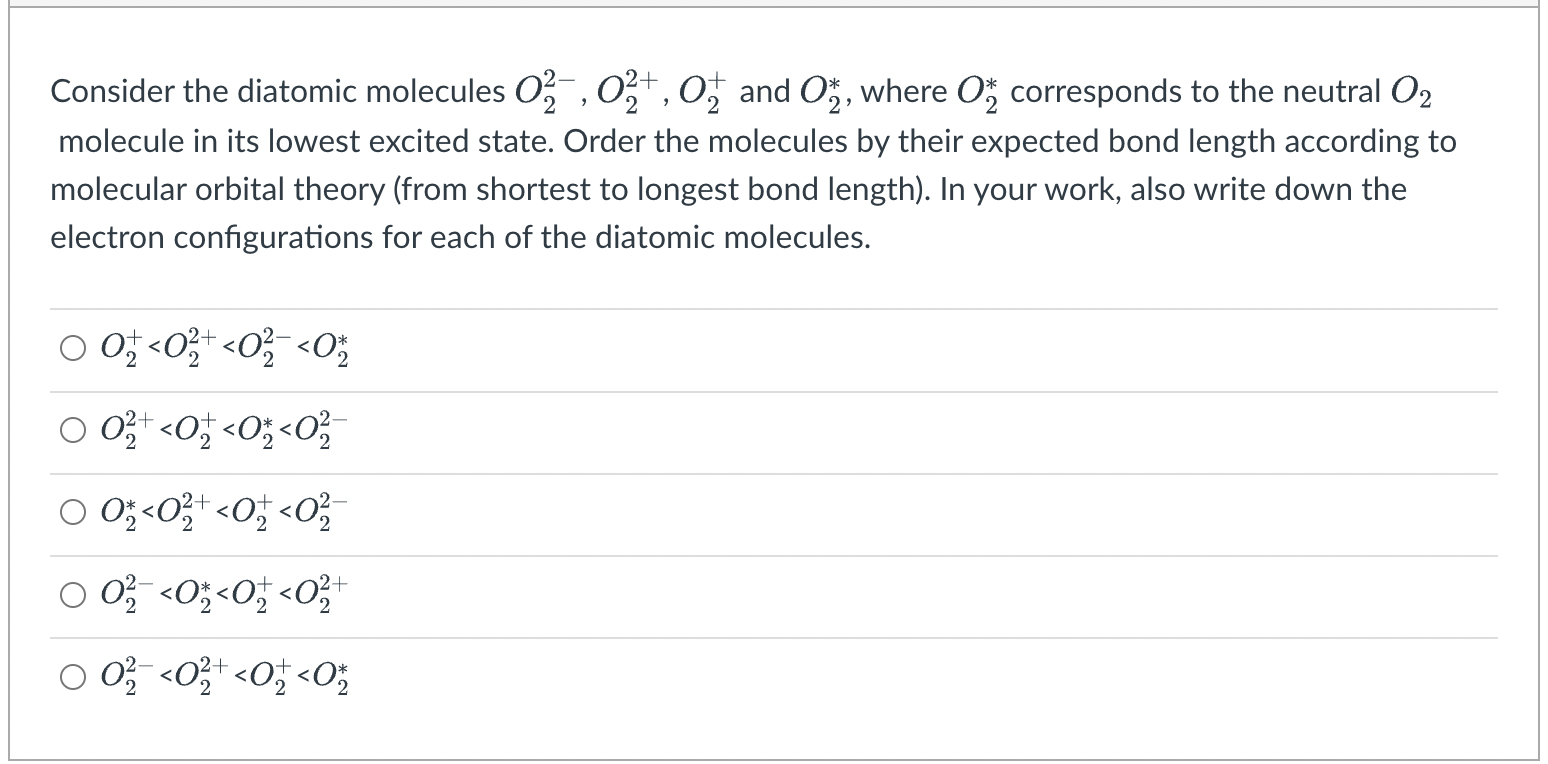
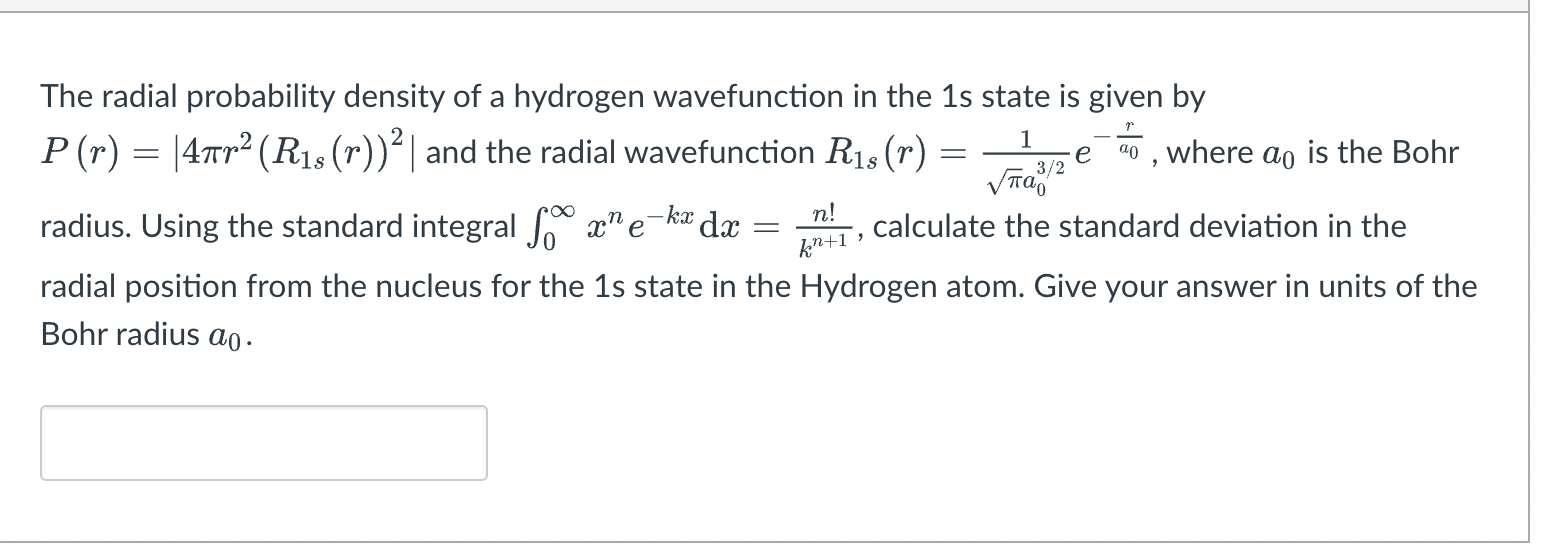
> Consider the diatomic molecules 02, 024, 07 and Om, where om corresponds to the neutral O2 molecule in its lowest excited state. Order the molecules by their expected bond length according to molecular orbital theory (from shortest to longest bond length). In your work, also write down the electron configurations for each of the diatomic molecules. + 00,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


