Question: Consider the following two reactions and the corresponding enthalpy changes: CH4(g)+2O2(g)CO2(g)+2H2O(l),H12CH4(g)+O2(g)2CH3OH(l),H2 Express the enthalpy change for the third reaction 2CH3OH(1)+3O2(g)2CO2(g)+4H2O(g),H3 in terms of H1 and
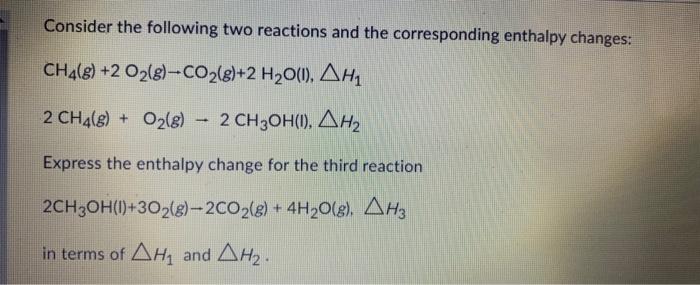
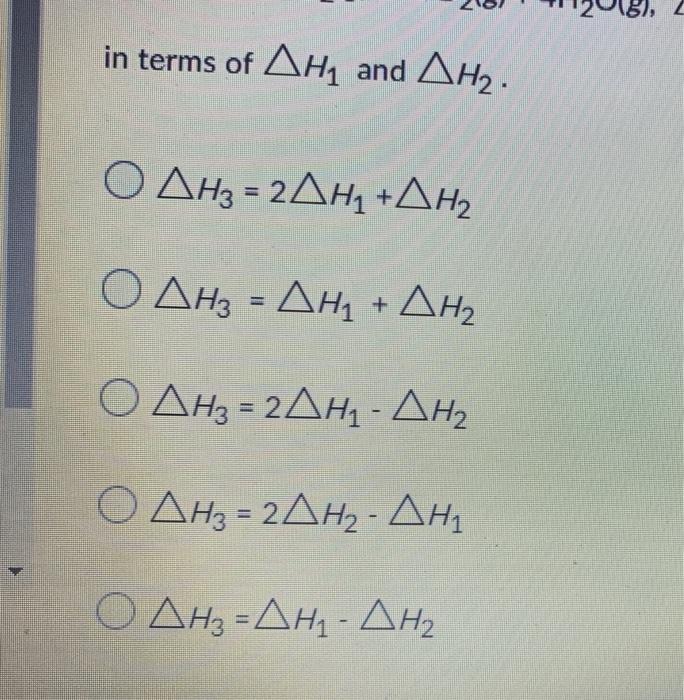
Consider the following two reactions and the corresponding enthalpy changes: CH4(g)+2O2(g)CO2(g)+2H2O(l),H12CH4(g)+O2(g)2CH3OH(l),H2 Express the enthalpy change for the third reaction 2CH3OH(1)+3O2(g)2CO2(g)+4H2O(g),H3 in terms of H1 and H2. intermsofH1andH2H3=2H1+H2H3=H1+H2H3=2H1H2H3=2H2H1H3=H1H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


