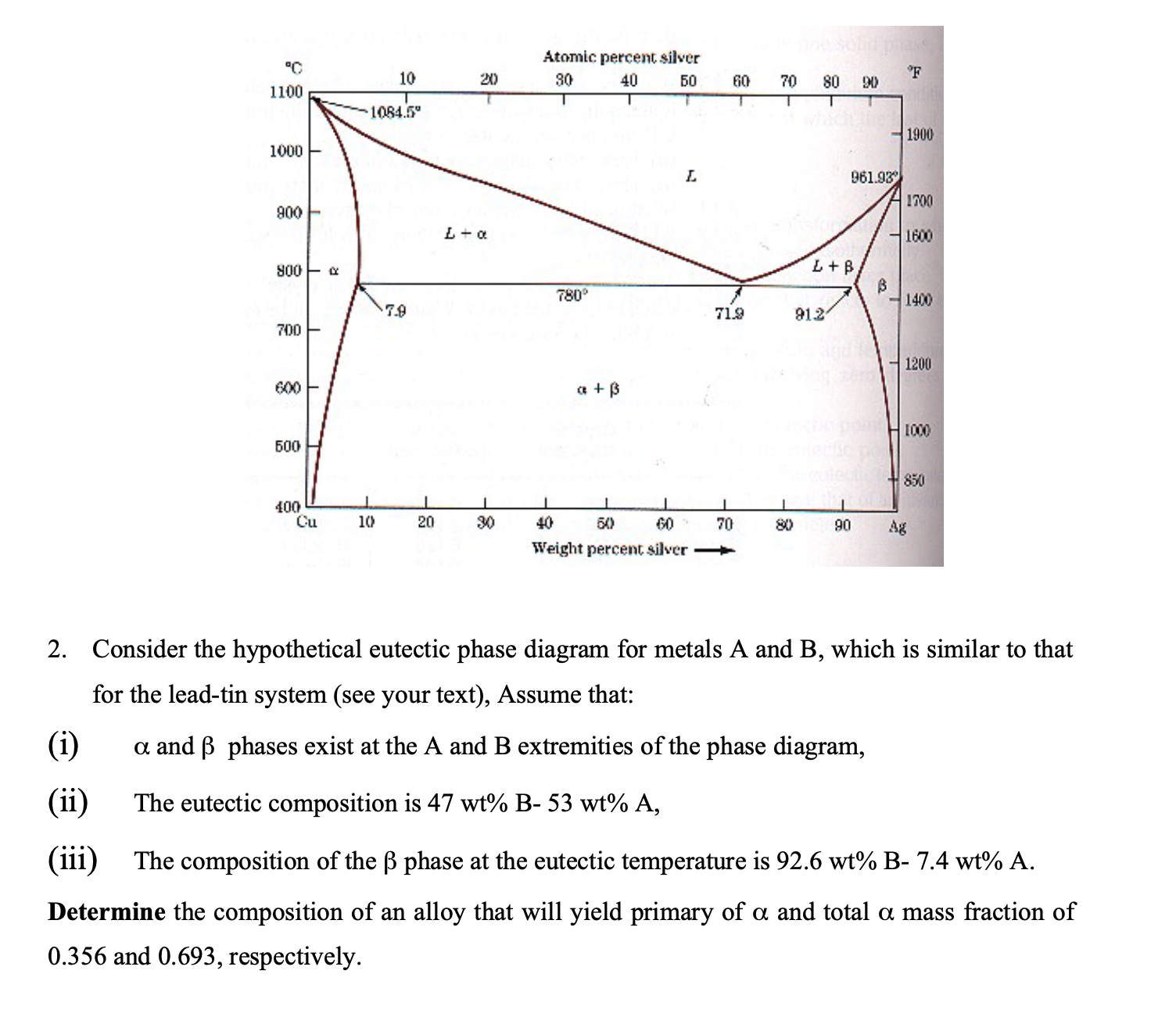
Question: Consider the hypothetical eutectic phase diagram for metals A and B , which is similar to that for the lead - tin system ( see
Consider the hypothetical eutectic phase diagram for metals A and which is similar to that
for the leadtin system see your text Assume that:
i and phases exist at the A and extremities of the phase diagram,
ii The eutectic composition is
iii The composition of the phase at the eutectic temperature is
Determine the composition of an alloy that will yield primary of and total mass fraction of
and respectively.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


