Question: Consider the hypothetical eutectic phase diagram for metals A and B, which is similar to that for the lead-tin system, (shown in the figure below.)
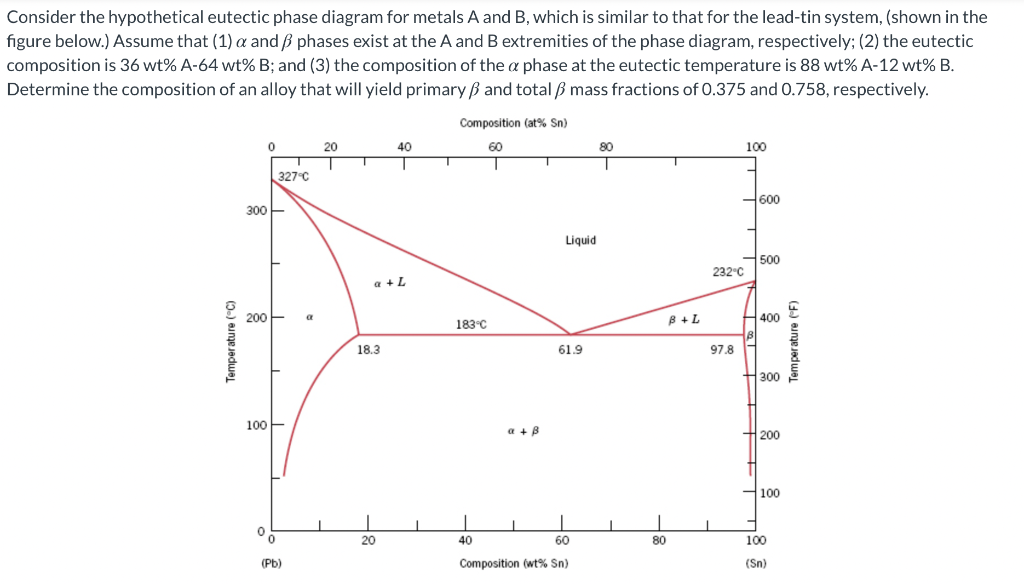
Consider the hypothetical eutectic phase diagram for metals A and B, which is similar to that for the lead-tin system, (shown in the figure below.) Assume that (1) a and phases exist at the A and B extremities of the phase diagram, respectively; (2) the eutectic composition is 36 wt% A-64 wt% B; and (3) the composition of the a phase at the eutectic temperature is 88 wt% A-12 wt% B. Determine the composition of an alloy that will yield primary and total mass fractions of 0.375 and 0.758, respectively. Composition (at% Sn) 0 20 60 80 100 40 1 327C 7600 300 Liquid 500 232C +L 200 a a 183C B+L 400 Temperature (C) emperature (F) 18.3 61.9 97.8 300 100 a+B 200 100 0 20 80 100 40 60 Composition (wt% Sn) (Pb) (Sn)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


