Question: Consider the mixing process shown in the attached figure. Two streams are mixed to produce one of the feeds for a chemical reactor. After mixing,
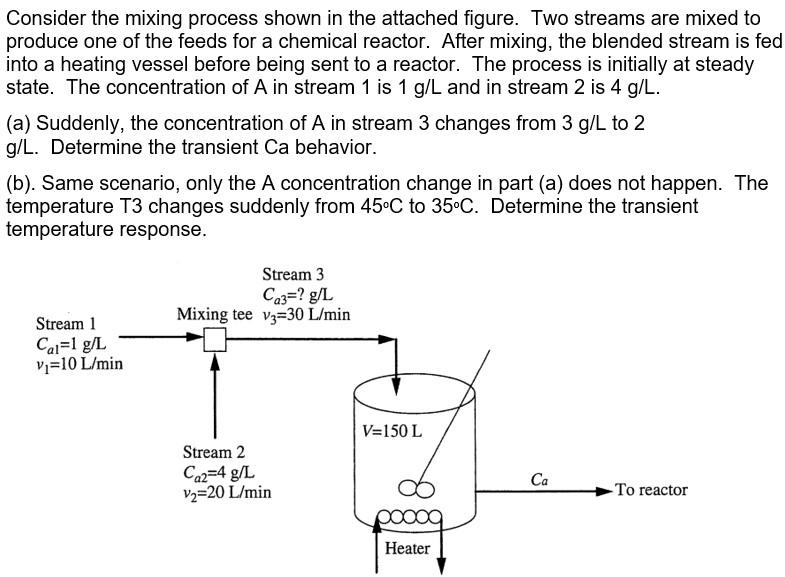
Consider the mixing process shown in the attached figure. Two streams are mixed to produce one of the feeds for a chemical reactor. After mixing, the blended stream is fed into a heating vessel before being sent to a reactor. The process is initially at steady state. The concentration of A in stream 1 is 1 g/L and in stream 2 is 4 g/L. (a) Suddenly, the concentration of A in stream 3 changes from 3 g/L to 2 g/L. Determine the transient Ca behavior. (b). Same scenario, only the A concentration change in part (a) does not happen. The temperature T3 changes suddenly from 45C to 35C. Determine the transient temperature response. Stream 3 Ca3=? g/L Mixing tee Vz=30 L/min Stream 1 Cal=1 g/L v;=10 L/min V=150 L Stream 2 Ca2=4 g/L Vz=20 L/min Ca To reactor Heater
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


