Question: Consider the following chemical mixing example. Two process streams are mixed to produce one of the feeds for the chemical reactor. After mixing, the blended
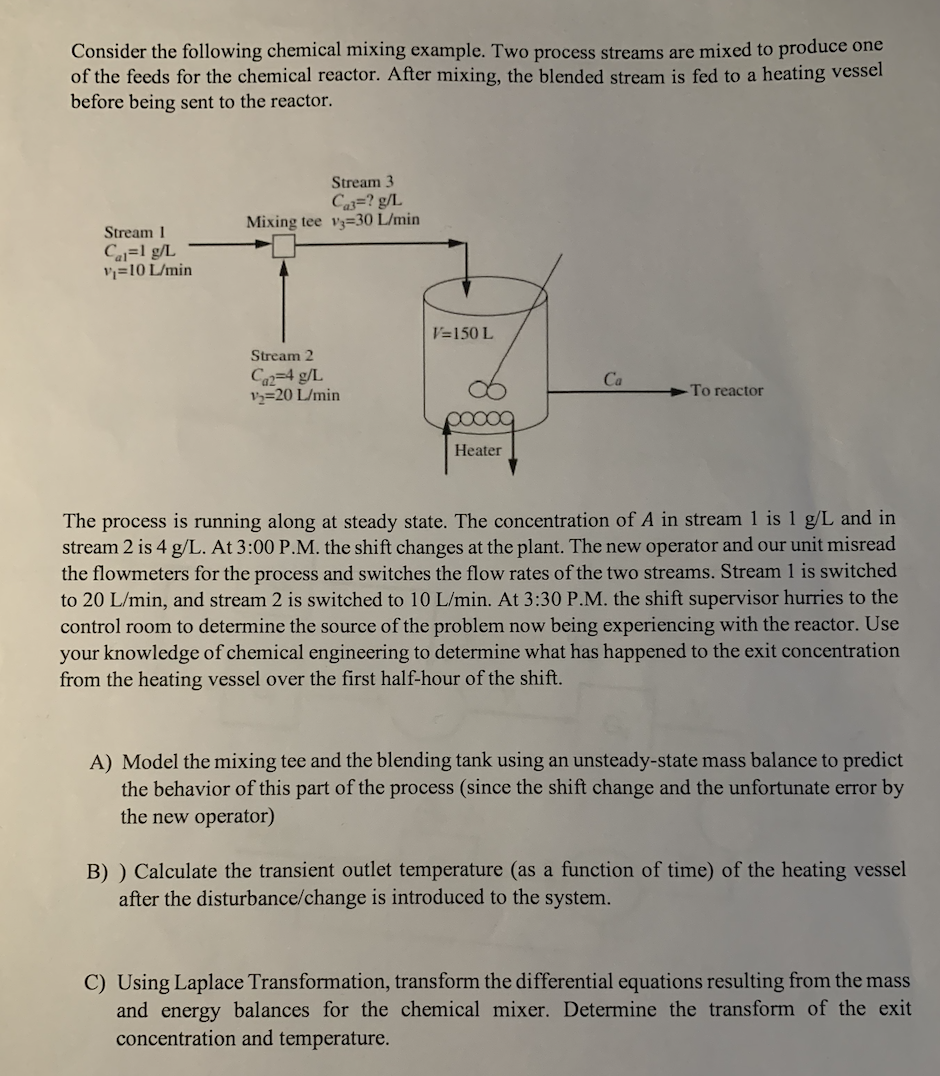
Consider the following chemical mixing example. Two process streams are mixed to produce one of the feeds for the chemical reactor. After mixing, the blended stream is fed to a heating vessel before being sent to the reactor. Stream 3 Car? g/L Mixing tee Vy=30 L/min Stream 1 Ca=1 g/L Vi=10 L/min V=150 L Stream 2 Cq24 g/L V>=20 L/min Ca To reactor 09 Heater The process is running along at steady state. The concentration of A in stream 1 is 1 g/L and in stream 2 is 4 g/L. At 3:00 P.M. the shift changes at the plant. The new operator and our unit misread the flowmeters for the process and switches the flow rates of the two streams. Stream 1 is switched to 20 L/min, and stream 2 is switched to 10 L/min. At 3:30 P.M. the shift supervisor hurries to the control room to determine the source of the problem now being experiencing with the reactor. Use your knowledge of chemical engineering to determine what has happened to the exit concentration from the heating vessel over the first half-hour of the shift. A) Model the mixing tee and the blending tank using an unsteady-state mass balance to predict the behavior of this part of the process (since the shift change and the unfortunate error by the new operator) B) ) Calculate the transient outlet temperature (as a function of time) of the heating vessel after the disturbance/change is introduced to the system. C) Using Laplace Transformation, transform the differential equations resulting from the mass and energy balances for the chemical mixer. Determine the transform of the exit concentration and temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


