Question: Consider the overall reaction system: CaCO3 (s) + CO2 (g) + H20 5 Ca2+ + 2 HCO3 with K = 1.5 x 10-6. What is
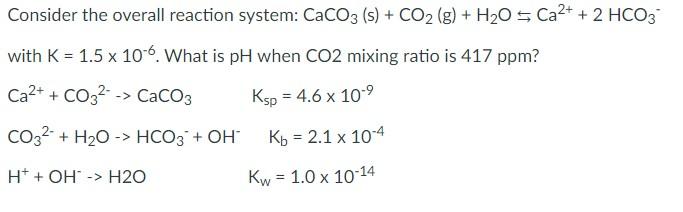
Consider the overall reaction system: CaCO3 (s) + CO2 (g) + H20 5 Ca2+ + 2 HCO3 with K = 1.5 x 10-6. What is pH when CO2 mixing ratio is 417 ppm? Ca2+ + CO32 -> CaCO3 Ksp = 4.6 x 10-9 CO32- + H2O -> HCO3 + OH Kb = 2.1 x 10-4 - H+ + OH -> H20 Kw = 1.0 x 10-14
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


