Question: Consider the reaction below: B A Reactant A decomposes to form two products, desired (B) and undesired (C) with activations energies of 7000 kcal/mole and
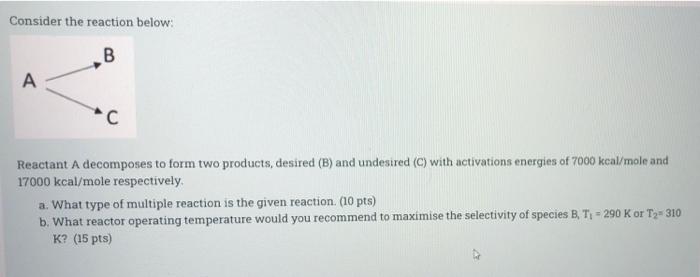
Consider the reaction below: B A Reactant A decomposes to form two products, desired (B) and undesired (C) with activations energies of 7000 kcal/mole and 17000 kcal/mole respectively a. What type of multiple reaction is the given reaction. (10 pts) b. What reactor operating temperature would you recommend to maximise the selectivity of species B, T = 290 K or Ty=310 K? (15 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


