Question: Example Reactant A decomposes by three simultaneous reactions to form three products, one that is desired B , and two that are undesired x and
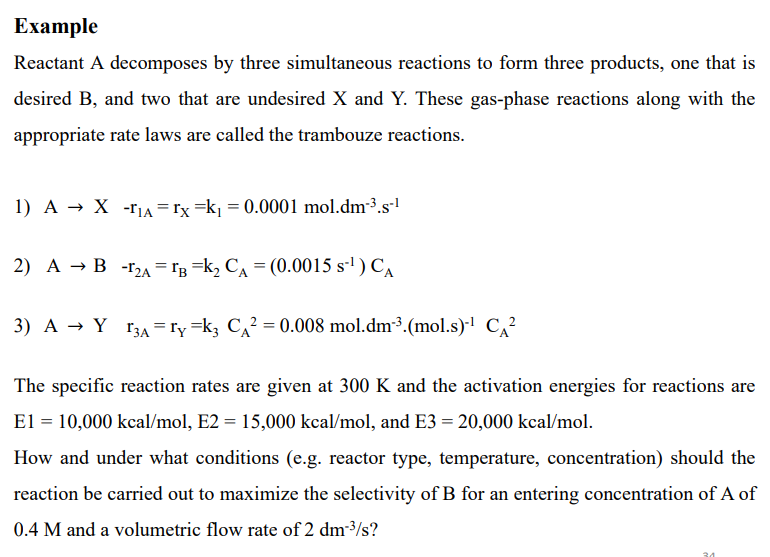
Example
Reactant A decomposes by three simultaneous reactions to form three products, one that is
desired and two that are undesired and These gasphase reactions along with the
appropriate rate laws are called the trambouze reactions.
mol.
mol.
The specific reaction rates are given at and the activation energies for reactions are
kcakca and kca
How and under what conditions eg reactor type, temperature, concentration should the
reaction be carried out to maximize the selectivity of for an entering concentration of of
and a volumetric flow rate of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


