Question: Consider the reaction below: Br2 (g) 2Br (g) K = 1.04 x 103 An 0.5 L vessel containing an equilibrium mixture of the gases
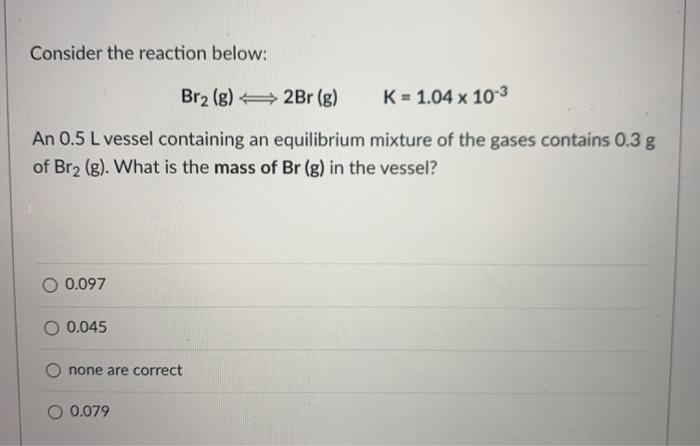
Consider the reaction below: Br2 (g) 2Br (g) K = 1.04 x 103 An 0.5 L vessel containing an equilibrium mixture of the gases contains 0.3 g of Br2 (g). What is the mass of Br (g) in the vessel? O 0.097 0.045 none are correct O 0.079
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Br 2 present in the vessel 03 g No of moles of Br 2 03 g 159808 g mol 1 000188 mol mole... View full answer

Get step-by-step solutions from verified subject matter experts


