Question: Consider the reaction profile shown below for a multi-step reaction with a net chemical equation AD : Fill in the blanks to make each of
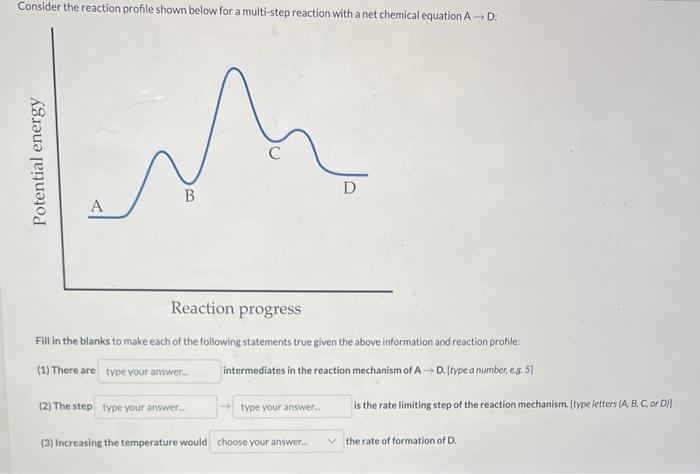
Consider the reaction profile shown below for a multi-step reaction with a net chemical equation AD : Fill in the blanks to make each of the following statements true given the above information and reaction profile: (1) There are intermediates in the reaction mechanism of AD. [type a mumber,es. 5] (2) The step is the rate limiting step of the reaction mechanism. [type letters (A, B, C, or D)] (3) Increasing the temperature would the rate of formation of D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


