Question: Consider the reaction shown below. OF2 + H20 + HF + O2 Which atom was oxidized in this reaction? [ Select ] Which atom was
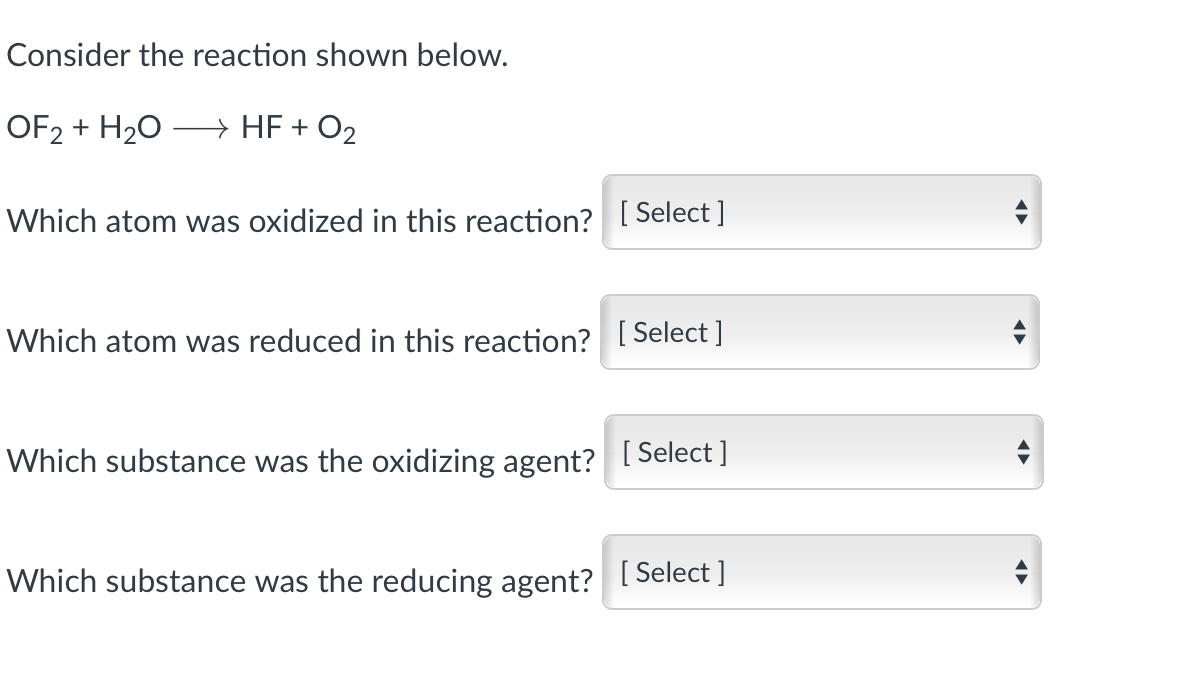
Consider the reaction shown below. OF2 + H20 + HF + O2 Which atom was oxidized in this reaction? [ Select ] Which atom was reduced in this reaction? [Select] Which substance was the oxidizing agent? [Select] Which substance was the reducing agent? [Select ]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


