Question: Consider the table below and answer the questions that follow: Atomic Radius Crystal Structure (nm) 0.1246 0.071 Element Ni H Ag Cr Co FCC
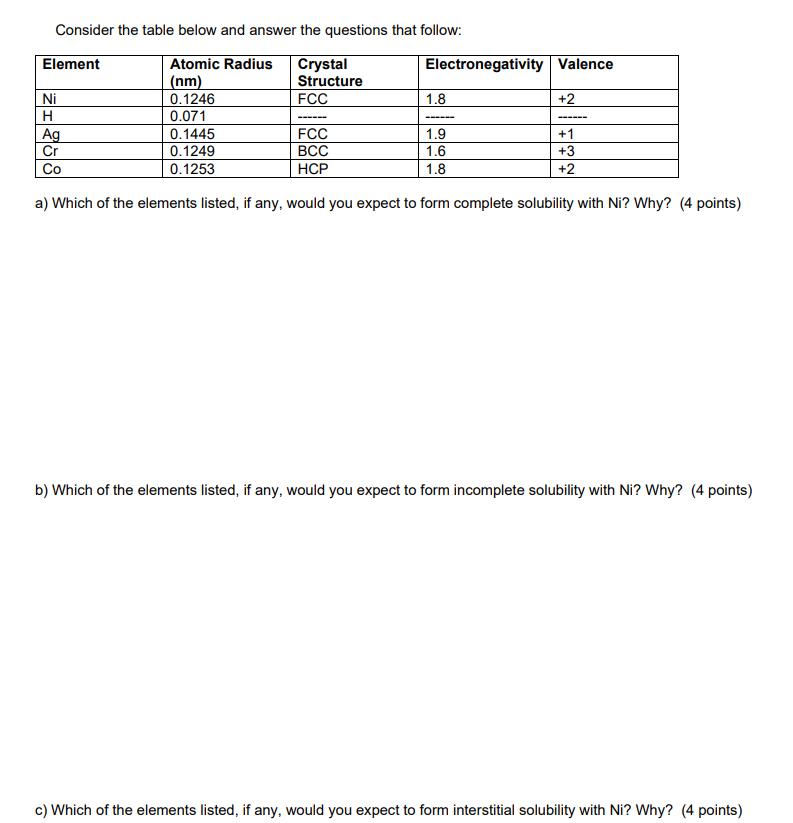
Consider the table below and answer the questions that follow: Atomic Radius Crystal Structure (nm) 0.1246 0.071 Element Ni H Ag Cr Co FCC 0.1445 0.1249 0.1253 ------ Electronegativity Valence 1.8 ---- +2 FCC 1.9 BCC 1.6 HCP 1.8 a) Which of the elements listed, if any, would you expect to form complete solubility with Ni? Why? (4 points) ------ +1 +3 +2 b) Which of the elements listed, if any, would you expect to form incomplete solubility with Ni? Why? (4 points) c) Which of the elements listed, if any, would you expect to form interstitial solubility with Ni? Why? (4 points)
Step by Step Solution
There are 3 Steps involved in it
Sure Here is my analysis of the table a Complete solubility Ni and Cr Both have the same crystal structure FCC and their atomic radii are similar 0124... View full answer

Get step-by-step solutions from verified subject matter experts


