Question: Consider this reaction: 201,0, () -- 201, () +50, () At a certain temperature it obeys this rate law. rate= (6.67 M's')[01,0) Suppose a vessel
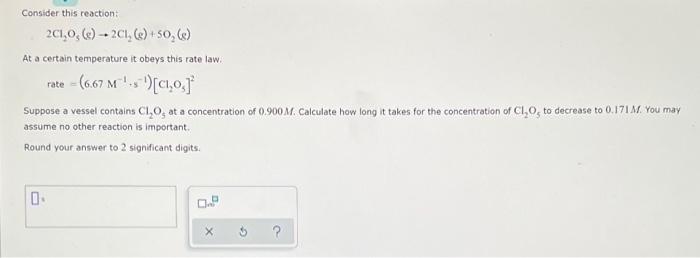
Consider this reaction: 201,0, () -- 201, () +50, () At a certain temperature it obeys this rate law. rate= (6.67 M's')[01,0) Suppose a vessel contains C1,0, at a concentration of 0.900 M. Calculate how long it takes for the concentration of Cl,0, to decrease to 0.171 M. You may assume no other reaction is important Round your answer to 2 significant digits. 0 0. 3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


