Question: Constants | Periodic Table Element X reacts with element Y to give a product containing X+ ions and Y- ions. You may want to
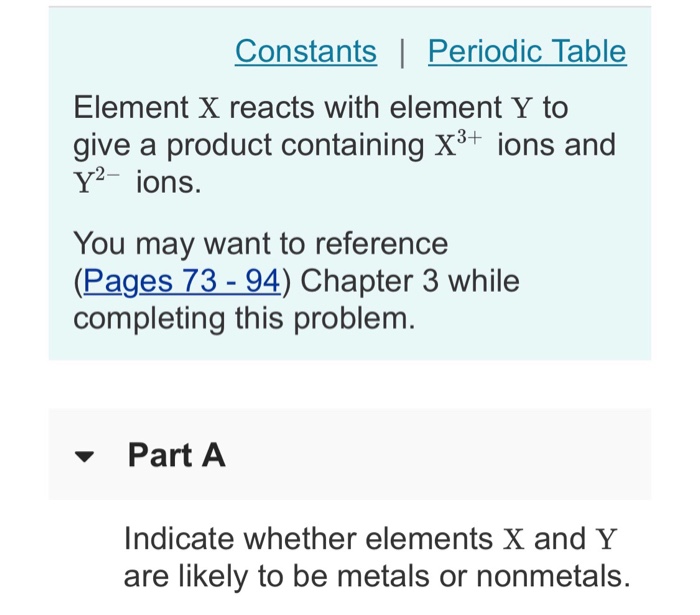
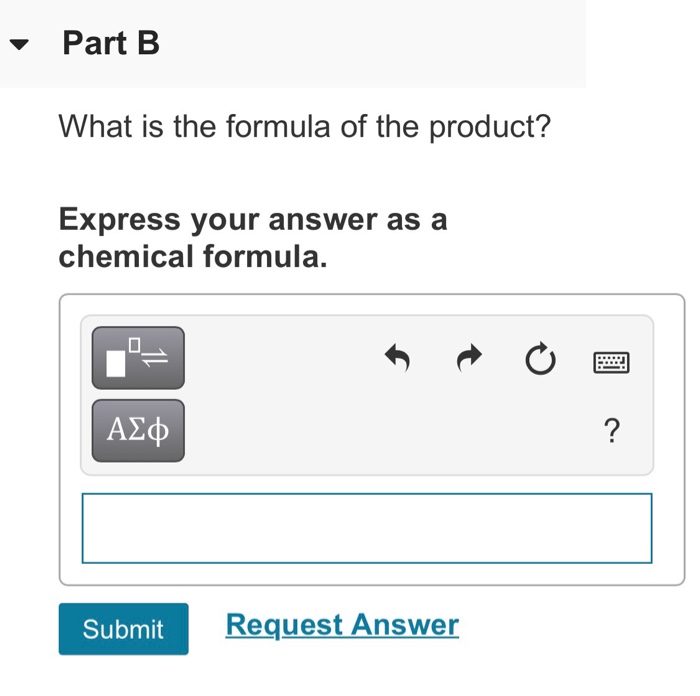
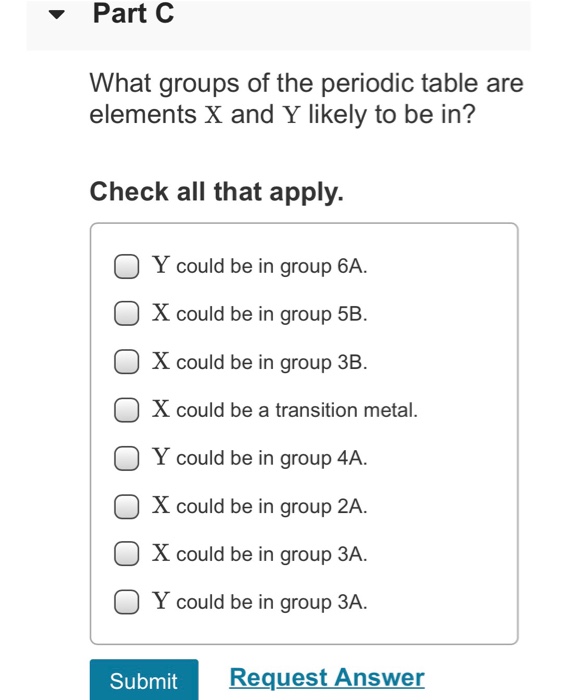
Constants | Periodic Table Element X reacts with element Y to give a product containing X+ ions and Y- ions. You may want to reference (Pages 73-94) Chapter 3 while completing this problem. Part A Indicate whether elements X and Y are likely to be metals or nonmetals. Part B What is the formula of the product? Express your answer as a chemical formula. Submit Request Answer ? Part C What groups of the periodic table are elements X and Y likely to be in? Check all that apply. Y could be in group 6A. X could be in group 5B. X could be in group 3B. X could be a transition metal. Y could be in group 4A. X could be in group 2A. X could be in group 3A. Y could be in group 3A. Submit Request Answer
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


