Question: Constants Periodic Table Part A Write chemical equations and corresponding equilibrium expressions for each of the two ionization steps of carbonic acid. Write chemical equations
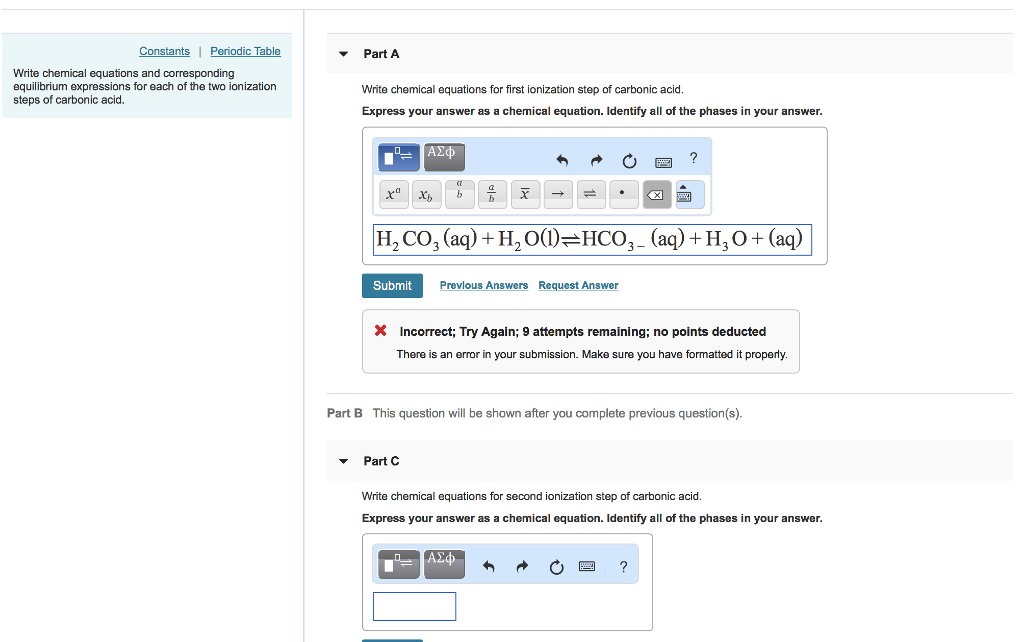
Constants Periodic Table Part A Write chemical equations and corresponding equilibrium expressions for each of the two ionization steps of carbonic acid. Write chemical equations for first ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your answer. [H2 CO, (aq) H20()-HCO,-(aq) + H30+ (aq) Submit Prevlous Answers Request Answer X Incorrect; Try Again; 9 attempts remaining; no points deducted There is an error in your submission. Make sure you have formatted it properly. Part B This question will be shown after you complete previous question(s) Part C Write chemical equations for second ionization step of carbonic acid. Express your answer as a chemical equation. Identify all of the phases in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


