Question: could you show your work with dimensional analysis A sample of vitamin A, C20H30O, contains 4.01024 atoms of carbon. How many moles of kitamin A
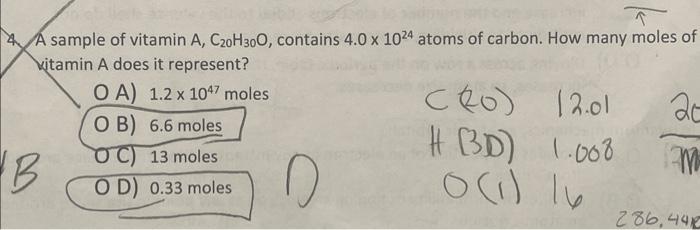
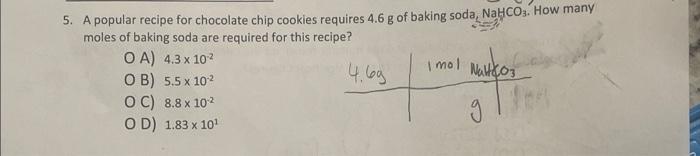

A sample of vitamin A, C20H30O, contains 4.01024 atoms of carbon. How many moles of kitamin A does it represent? O A) 1.21047 moles B) 6.6 moles [(20)12.01 C) 13 moles H(3D) 1.008 D) 0.33 moles (1)l286,44 5. A popular recipe for chocolate chip cookies requires 4.6g of baking soda, NaHCO3. How many moles of baking soda are required for this recipe? A) 4.3102 B) 5.5102 C) 8.8102 D) 1.83101 12. (8 pts) In scientific work, density is often reported in g/cm3, but engineers historically use the unit pounds per cubic foot (lb/ft3). Given that the density of aluminum at room temperature is 2.70g/cm3, what is its density in lb/ft3 ? 1.000lb=0.4536kg. 1 inch =2.54cm exactly. 1ft=12 inches. (8 pts) UV light from the sun is strongest during mid-day. It also takes a mere 26J/cm2 of UV fadiation (delivered in just 15 mins) to develop skin damage. If you sit out by the pool, how many photons can you absorb before developing skin damage? Assume that 1.5104cm2of skin is exposed, without sunscreen, in your sitting position ( 1% of total skin area) and that the average wavelength of UV light is 360nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


