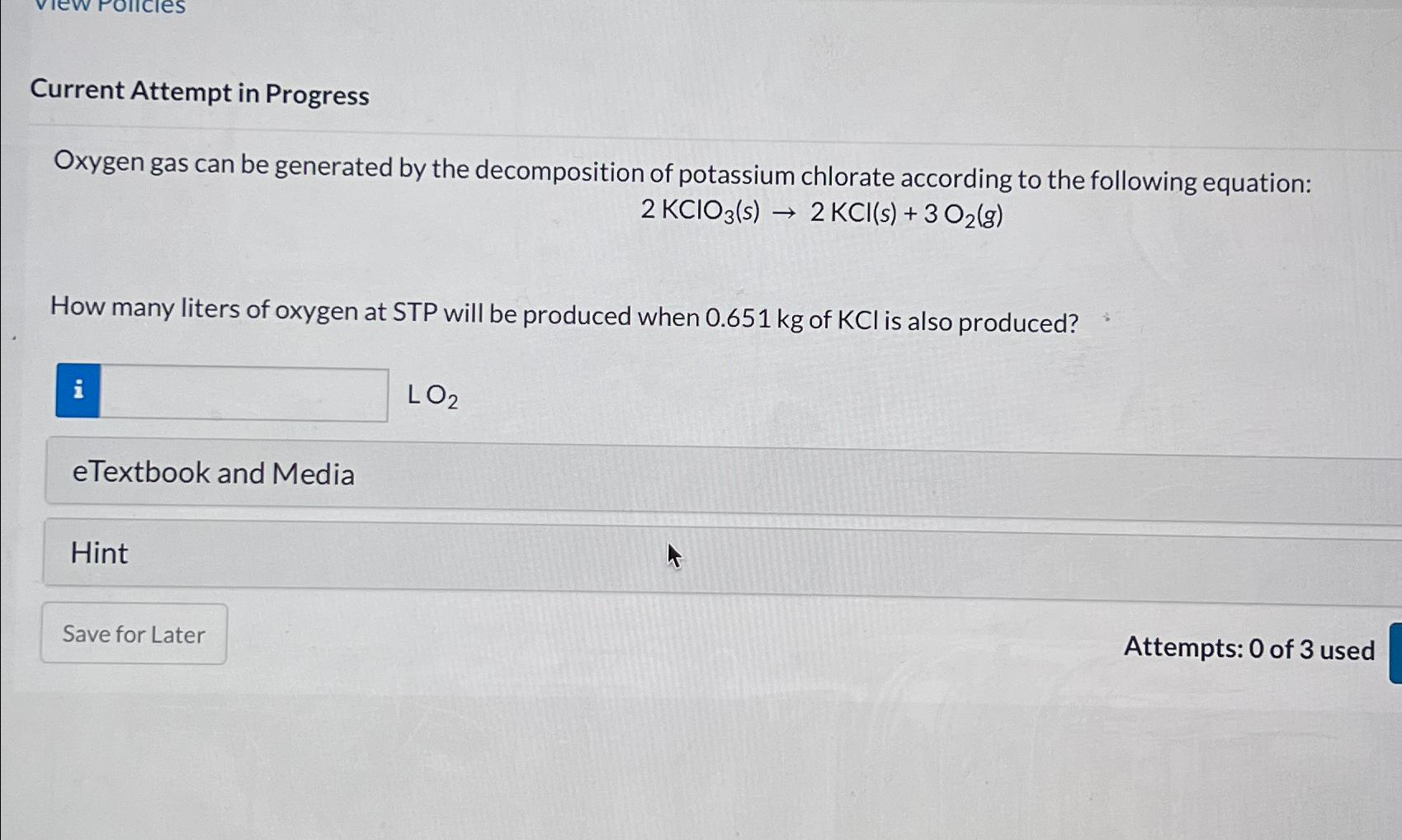
Question: Current Attempt in Progress Oxygen gas can be generated by the decomposition of potassium chlorate according to the following equation: 2 K C l O
Current Attempt in Progress
Oxygen gas can be generated by the decomposition of potassium chlorate according to the following equation:
How many liters of oxygen at STP will be produced when of is also produced?
i
eTextbook and Media
Hint
Attempts: of used
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


