Question: Data Analysis 1. Plot log10 (O - O) versus t for 1 M HCl and 2 M HCl runs. Is first order behavior observed? 2.

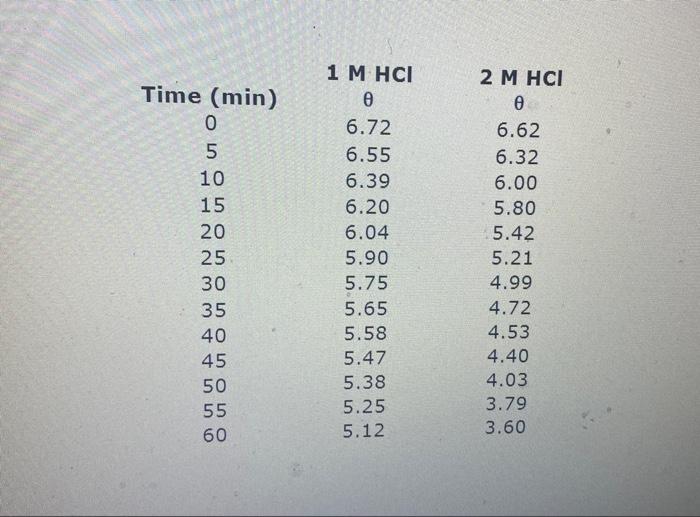
Data Analysis 1. 2 kak 3. 4. 5. Plot log10 (0-0.) versus t for 1 M HCl and 2 M HCl runs. Is first order behavior observed? Calculate kobs for the 1 M HCl and 2M HCl runs. Is kobs proportional to [H]? Calculate k2 for the 1 M HCI and 2 M HCl runs. Comment on the magnitude of your result List possible sources of error. e should be - 2.09 Prove this. Explain why eis -2.0 instead of O? Why is this known as a pseudo first order reaction? Explain using appropriate equations Briefly describe operation of the polarimeter (Include following terms in your description: light source, unpolarized light. Nicol prism polarizer, plane-polarired beam, optically active compounds, angle of rotation analyzer 6. 7 Time (min) O LO 5 10 15 20 25 30 35 40 45 50 55 60 1 M HCI 0 6.72 6.55 6.39 6.20 6.04 5.90 5.75 5.65 5.58 5.47 5.38 5.25 5.12 2 M HCI 6.62 6.32 6.00 5.80 5.42 5.21 4.99 4.72 4.53 4.40 4.03 3.79 3.60
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


