Question: Data Collection and Analysis: Part I Data Table: Mass of Cu moles Cu Part I Observations Part 1 Balanced Equation (hint: use 4 moles
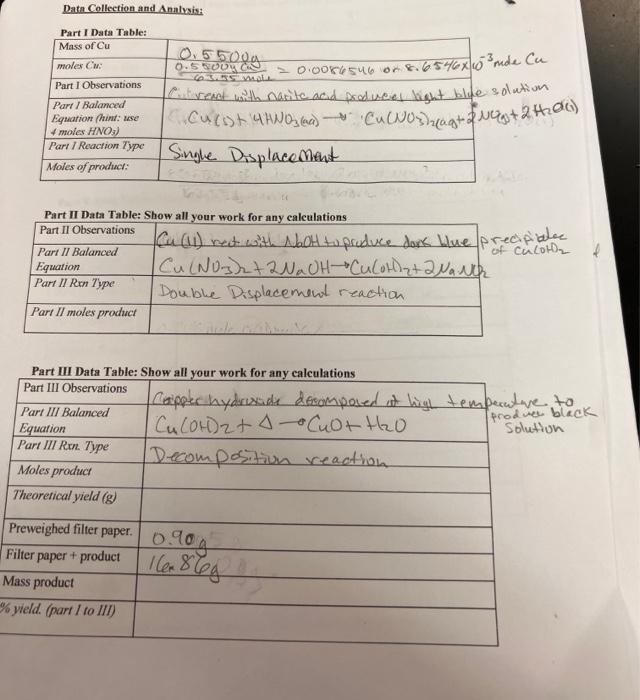
Data Collection and Analysis: Part I Data Table: Mass of Cu moles Cu Part I Observations Part 1 Balanced Equation (hint: use 4 moles HNO3) Part 1 Reaction Type Moles of product: Part II Balanced Equation Part II Ren Type Part II moles product Part II Data Table: Show all your work for any calculations Part II Observations 0.55009 0.55000 Part III Balanced Equation Part III Rxn. Type Moles product Theoretical yield (g) 6.3.55 male = 0.0086546 or 8.6546x 10 mde Cu Cill react with narite and produces light blue solation Curst 47W03 (69) Cu(NO3)2(ag+2 Ng+2+0) - Single Displacement Preweighed filter paper. Filter paper + product Mass product % yield. (part I to III) Part III Data Table: Show all your work for a Part III Observations Cu (11) nect with NaOH to produce dark blue precipiales Cu(NO3)2 + 2NaOH-*Culor+2 NaNk of Culot Double Displacement reaction. any calculations Capper hydrixide decomposed at high temperature to Cu (01)2 + (0+ t20 produce black Solution Decomposition reaction 0.90g 166 868
Step by Step Solution
3.36 Rating (152 Votes )
There are 3 Steps involved in it
The detailed answers are given as PartI The equation Cus 4 HNO3 aq CuNO32aq2 NO2g2 H2Ol We know that ... View full answer

Get step-by-step solutions from verified subject matter experts


