Question: Show all your work and keep significant figures in mind through the problem. Thank you in advance. On the equation below please disregard the :
Show all your work and keep significant figures in mind through the problem. Thank you in advance. On the equation below please disregard the : and .
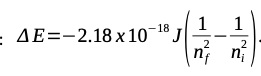

- AE=-2.18 x 10-18 J 1 2 nf 2 n a. Calculate the energy required to ionize a hydrogen atom using the Rydberg Equation above. b. What wavelength of light would be required to ionize a hydrogen atom? C. In what region of the electromagnetic spectrum does the light emission occur?
Step by Step Solution
There are 3 Steps involved in it
To solve these problems well use the provided Rydberg formula Delta E 218 times 1018 textJ left frac... View full answer

Get step-by-step solutions from verified subject matter experts


