Question: Data Table 2: Borax Data begin{tabular}{|c|c|c|c|c|} hline Buffer & pH & pH 5 & pH1 & pH 20mL Borax hline Water & 6.9 &
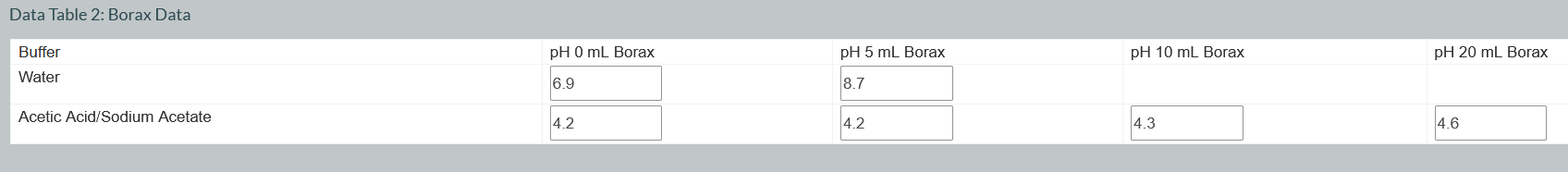



Data Table 2: Borax Data \begin{tabular}{|c|c|c|c|c|} \hline Buffer & pH & pH 5 & pH1 & pH 20mL Borax \\ \hline Water & 6.9 & 8.7 & & \\ \hline Acetic Acid/Sodium Acetate & 4.2 & 4.2 & 4.3 & 4.6 \\ \hline \end{tabular} Data Table 1: Cola Data \begin{tabular}{|c|c|c|c|c|} \hline \multirow{2}{*}{\begin{tabular}{l} Buffer \\ Water \end{tabular}} & pH 0 mL Cola & pH 5 mL Cola & \multirow[t]{2}{*}{ pH 10 mL Cola } & \multirow[t]{2}{*}{ pH 20mL Cola } \\ \hline & 7 & 3.3 & & \\ \hline Acetic Acid/Sodium Acetate & 4.2 & 4.7 & 4.2 & 4.2 \\ \hline \end{tabular} Did distilled water act as a buffer in the experiment? How do the results in Data Tables 1 and 2 support the role of a buffer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


