Question: Please help me with exercise 1 and 2 Data: Table 1: Addition of strong acid to Buffer A Table 2: Addition of strong acid to
 Please help me with exercise 1 and 2
Please help me with exercise 1 and 2
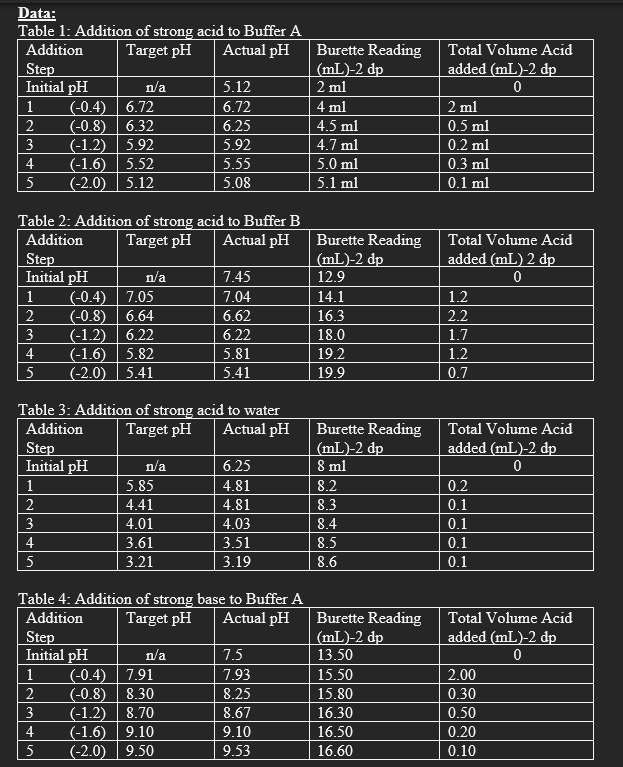
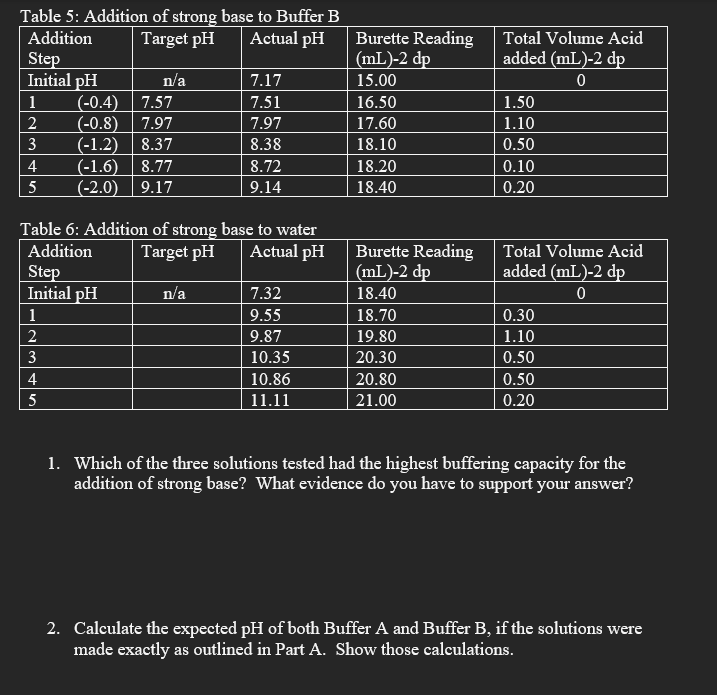
Data: Table 1: Addition of strong acid to Buffer A Table 2: Addition of strong acid to Buffer B Table 3: Addition of strong acid to water \begin{tabular}{|l|c|l|l|l|} \hline AdditionStep & Target pH & Actual pH & BuretteReading(mL)2dp & TotalVolumeAcidadded(mL)2dp \\ \hline Initial pH & n/a & 6.25 & 8ml & 0 \\ \hline 1 & 5.85 & 4.81 & 8.2 & 0.2 \\ \hline 2 & 4.41 & 4.81 & 8.3 & 0.1 \\ \hline 3 & 4.01 & 4.03 & 8.4 & 0.1 \\ \hline 4 & 3.61 & 3.51 & 8.5 & 0.1 \\ \hline 5 & 3.21 & 3.19 & 8.6 & 0.1 \\ \hline \end{tabular} Table 4: Addition of strong base to Buffer A \begin{tabular}{|l|c|l|l|l|l|} \hline AdditionStep & Target pH & Actual pH & BuretteReading(mL)-2dp & TotalVolumeAcidadded(mL)-2dp \\ \hline \multicolumn{1}{|l|}{ Initial pH} & n/a & 7.5 & 13.50 & \multicolumn{1}{c|}{0} \\ \hline 1 & (0.4) & 7.91 & 7.93 & 15.50 & 2.00 \\ \hline 2 & (0.8) & 8.30 & 8.25 & 15.80 & 0.30 \\ \hline 3 & (1.2) & 8.70 & 8.67 & 16.30 & 0.50 \\ \hline 4 & (1.6) & 9.10 & 9.10 & 16.50 & 0.20 \\ \hline 5 & (2.0) & 9.50 & 9.53 & 16.60 & 0.10 \\ \hline \end{tabular} Table 6: Addition of strong base to water 1. Which of the three solutions tested had the highest buffering capacity for the addition of strong base? What evidence do you have to support your answer? 2. Calculate the expected pH of both Buffer A and Buffer B, if the solutions were made exactly as outlined in Part A. Show those calculations. Part A 1) prepare 100mLs of a 0.1M solution of KH2PO4. (It is a dry solid) 2) prepare Buffer A a) in a 100mL beaker, pipette: 20.0mL of 0.1MKH2PO4 20.0mL of 0.1MNaOH b) in another 100mL beaker, pipette: 20.0mL of cooled distilled water 20.0mL of 0.1MKH2PO4 c) pour solution a) into the beaker containing solution b) 3) prepare Buffer B in a 100mL beaker, add by pipette: 40.0mL of 0.1MKH2PO4 10.0mL of cooled distilled water 30.0mL of 0.1MNaOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


