Question: Densitity for solution - 1 F 1 2 40 m B. DETERMINATION OF AH.. FOR THE REACTION OF NaOH AND HCI Trial 1 Trial 2
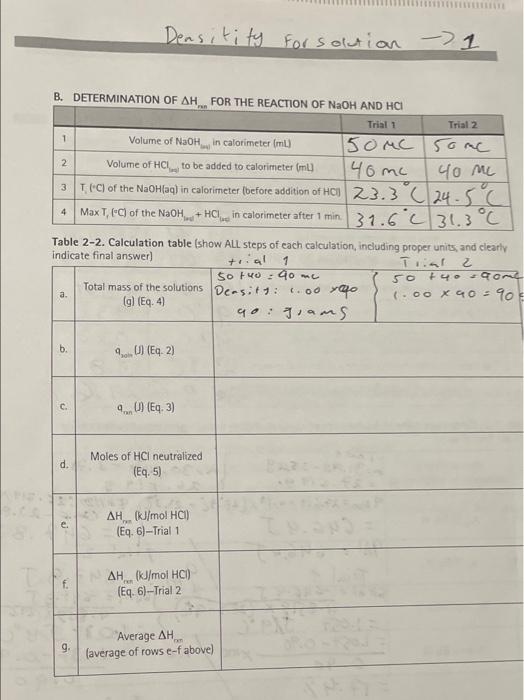


Densitity for solution - 1 F 1 2 40 m B. DETERMINATION OF AH.. FOR THE REACTION OF NaOH AND HCI Trial 1 Trial 2 Volume of NaOH in calorimeter (ml) 150MC some Volume of HCL to be added to calorimeter (L) 40 me 3 (C) of the NaOHlaq) in calorimeter (before addition of HCI 23.3 C 24.5C 4 Max T, (C) of the NaOH, + HCl in calorimeter after 1 min. 31.6231.3C Table 2-2. Calculation table (show ALL steps of each calculation, including proper units, and clearly indicate final answer 1 Trial 2 so tuo 90 me 50 +4-O Total mass of the solutions Densitt: 1.00 vafo (.00 X 40 = 90 (g) (Eq. 4 go: grams trial a. b. (E. 2) 4. U (E9.3) d d. Moles of HCl neutralized (E9.5) AH. (kJ/mol HCI) (Eq. 6)-Trial 1 AH(kJ/mol HCI) (Eq. 6) --Trial 2 g "Average AH (average of rows e-f above) Calculate then using Equation (?). = specific heat. x massXAT = 4.184 Vg" 52.71 8 x 13.3C (E-2) 2933.16J Determine cours using Equation (3) (Eq 0--0) Therefore: com ) - ) oi = 2933.16 J = 2,913 kg Determine the moles of KOH (using the atomic mass of KOH). mole = 2.71 g KOHX 1 mole KOH 0.0483 mol KOH 56.11 g KOH Then determine AH..in kJ/mol KOH, using Eqution (5) .. = 4. ( mol KOH AH.. -2.933 kJ 0.0483 mol KOH . =-60.7 kJ/mol KOH (E4.5) (E46) X 100% In This Experiment In Part A of this experiment, the system is the aluminum rods and the surroundings are the water. The specific heat of aluminum will be determined. The percent error will also be determined using the literature specific heat of Al in Equation (6). Accepted specific heat of Al (from literature) - 0.90 J/gC. Experimental value of specific heat of Al is what you calculate from this experiment. % error ("experimental value"-"accepted value") (Eq.7) "accepted value" In Part B, the system is the acid/base reaction and the aqueous solution is the surroundings. The mass of the solution equals the combined total mass of HCl and NaOH solutions. Use the density to determine the mass. Density of HCI=1.19 g/mL Density of NaOH = 1.515 g/mL Note: For simplicity, you have the option to assume the density of HCl and NaOH are each equal to water, which is 1.000 g/mL. If you chose to do this, then be consistent throughout your calculations. DETERMINATION OF SPECIFIC HEAT AND VARIOUS HEATS OF REACTIONS masso SOLUTION First you must determine the mass of the water in the calorimeter using the density Equation (4) .(g) = mbyo density, (Eq. 4) Knowns: Volume of water in this example is 30,0 mL Density of water is 1.000 g/mL. Unknown: Mass of water (g) Therefore: mass 1,0 = 30.0 mL. x 1.000 g/mL = 30.0 g Next: The Fe is the system and the water is the surrounding. Calculate the cousing Equation (2). 4,0 = specific heat,o x masso X AT10 (Eq.2) AT -T.-T... = 28.3C - 22.0C = 6.3C 41,0 = 4.184 J/g*C 30.0 g * 6.3C ano = 790.0J Then: Determine 4s, using Equation (3). yem (I) = -4urrounding ) (Eq. 3) Therefore: 420) = -40 (1) "=-790.0 J Finally: Determine the specific heat of Fe, using Equation (1). 4J).. specific heatre (J/gC) = (mass (g). XAT("C).] (Eq. 1) -790.0) Therefore: specific heat. (J/gC) = [24.3 g X (28.3C -100.00C) specific heat, U/gC) = 0.45 J/g C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


