Question: we will derive the Maxwell-Boltzmann speed distribution P(v) for molecules with m my mass m at temperature T, P(v) = 4, v exp where
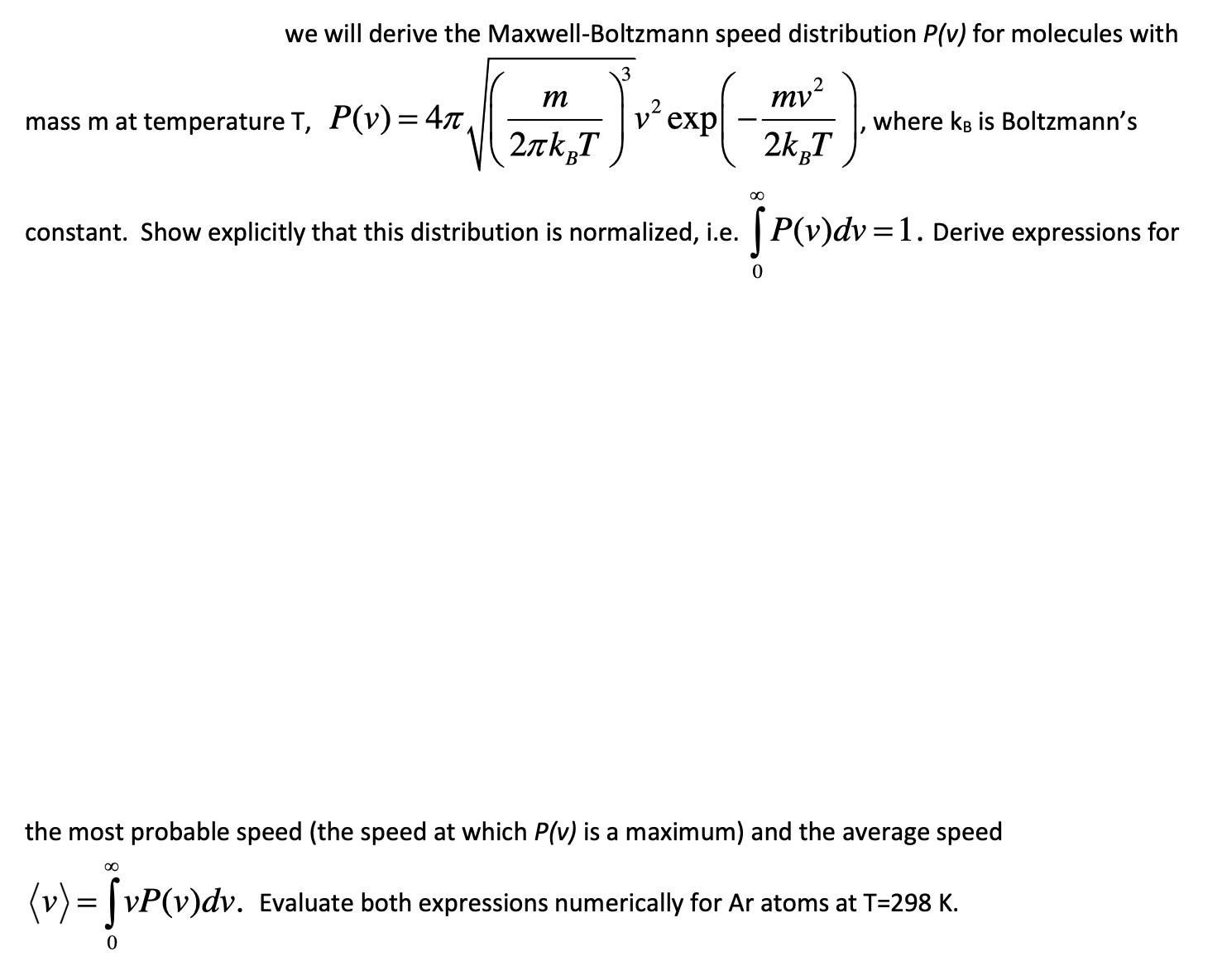
we will derive the Maxwell-Boltzmann speed distribution P(v) for molecules with m my mass m at temperature T, P(v) = 4, v exp where kB is Boltzmann's 2kBT 2kBT 00 constant. Show explicitly that this distribution is normalized, i.e. [P(v)dv = 1. Derive expressions for 0 the most probable speed (the speed at which P(v) is a maximum) and the average speed 00 (v) = vP(v)dv. 0 Evaluate both expressions numerically for Ar atoms at T=298 K.
Step by Step Solution
3.42 Rating (149 Votes )
There are 3 Steps involved in it
To show that theMaxwellBoltzmannspeed distribution is normalized we need to evaluate the integral of ... View full answer

Get step-by-step solutions from verified subject matter experts


