Question: Determine A's Concentration in (M) in two significant figures The following reaction follows first-order kinetics with a half-life of 0.321s. AB+C Initially, a vessel contains
Determine A's Concentration in (M) in two significant figures 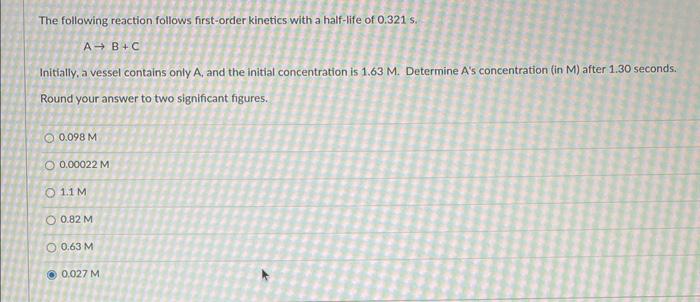

The following reaction follows first-order kinetics with a half-life of 0.321s. AB+C Initially, a vessel contains only A, and the initial concentration is 1.63M. Determine A's concentration (in M ) after 1.30 seconds. Round your answer to two significant figures. 0.098M0.00022M1.1M0.82M0.63M0.027M
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


