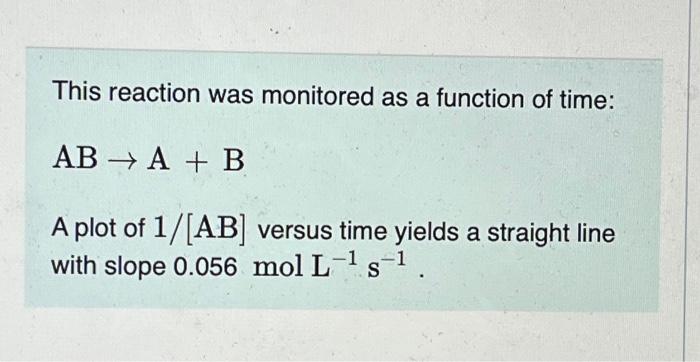
Question: please help This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope 0.056molL1s1.
![ABA+B A plot of 1/[AB] versus time yields a straight line with](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8f324ec65f_20466f8f32490efc.jpg)

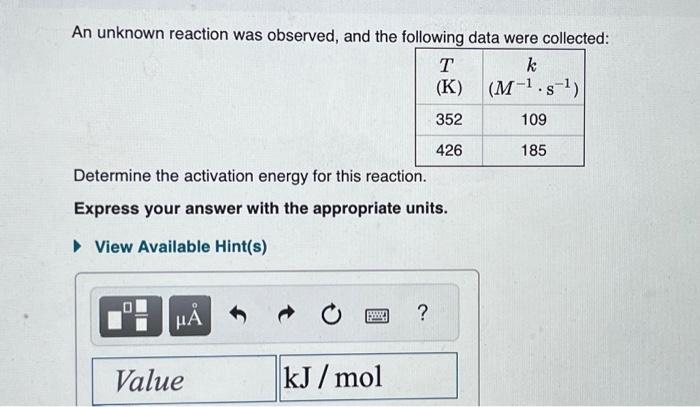
This reaction was monitored as a function of time: ABA+B A plot of 1/[AB] versus time yields a straight line with slope 0.056molL1s1. If the initial concentration of AB is 0.230molL1, and the reaction mixture initially contains no products, what are the concentrations of A and B after 80s ? Express your answers using two significant figures separated by a comma. A certain reaction has an activation energy of 70.0kJ/mol and a frequency factor of A1=3.601012M1s1. What is the rate constant, k, of this reaction at 29.0C ? Express your answer with the appropriate units. Indicate the multiplication of units explicitly either with a multiplication dot (asterisk) or a dash. An unknown reaction was observed, and the following data were collected: Determine the activation energy for this reaction. Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


