Question: Determine the angular momentum L at 3d state. A. V2h B. V6h C. 3h D. 5hn=1 beam electron Incident A d sine J d =

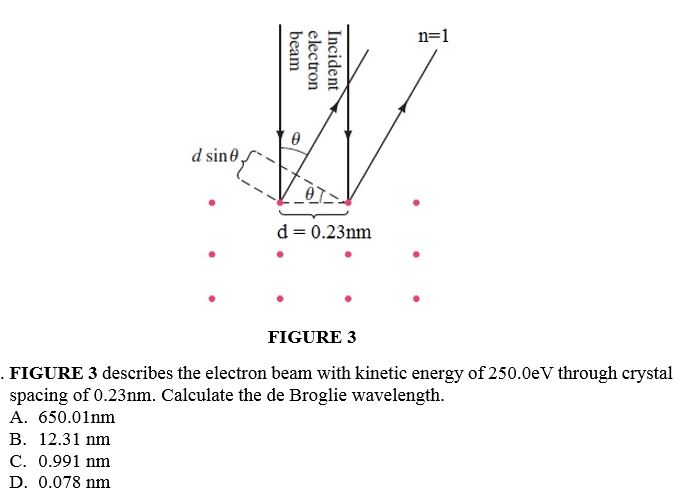

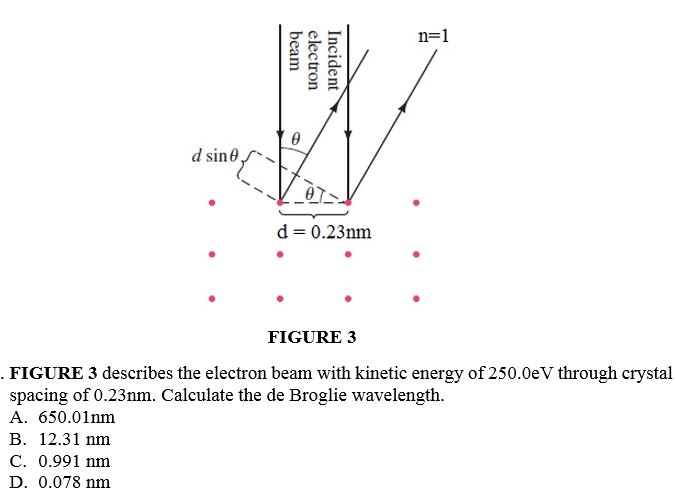
Determine the angular momentum L at 3d state. A. V2h B. V6h C. 3h D. 5hn=1 beam electron Incident A d sine J d = 0.23nm . FIGURE 3 FIGURE 3 describes the electron beam with kinetic energy of 250.0eV through crystal spacing of 0.23nm. Calculate the de Broglie wavelength. A. 650.01nm B. 12.31 nm C. 0.991 nm D. 0.078 nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


