Question: Determine the conditional formation constant (K) for a solution containing 7.50 103 M Cay2-, 1.50 x 10-8 M CaSO4, and 1.00 x 10-5 M
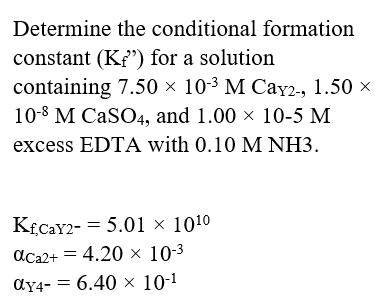
Determine the conditional formation constant (K") for a solution containing 7.50 103 M Cay2-, 1.50 x 10-8 M CaSO4, and 1.00 x 10-5 M excess EDTA with 0.10 M NH3. KCAY2- = 5.01 1010 aCa2+ = 4.20 10-3 ay4- = 6.40 x 10-1
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


