Question: Determine the ff. if true or false. Support it with an explanation 1. Excess Reactant is the reactant in a chemical reaction that remains when
Determine the ff. if true or false. Support it with an explanation
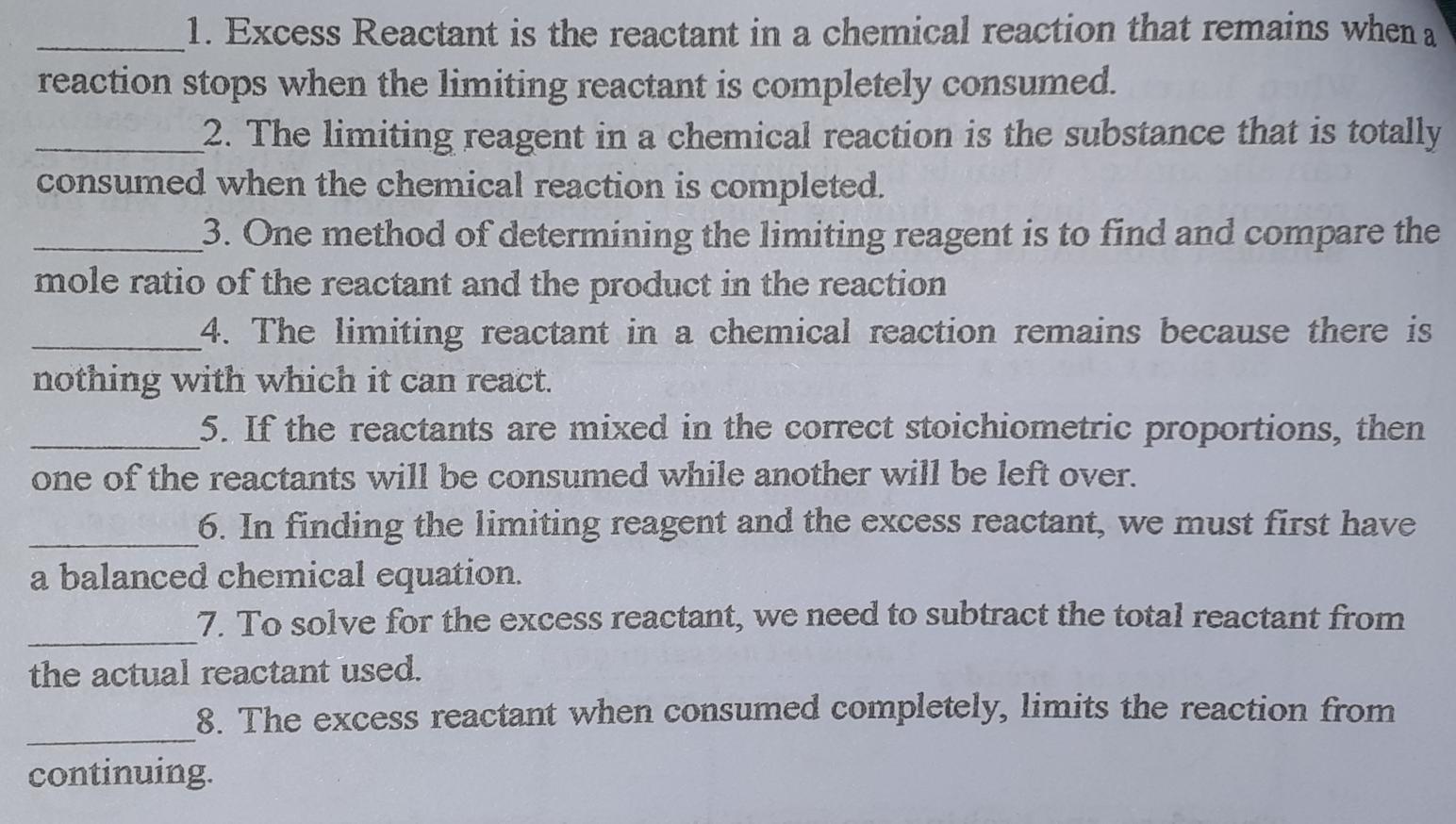
1. Excess Reactant is the reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed. 2. The limiting reagent in a chemical reaction is the substance that is totally consumed when the chemical reaction is completed. 3. One method of determining the limiting reagent is to find and compare the mole ratio of the reactant and the product in the reaction 4. The limiting reactant in a chemical reaction remains because there is nothing with which it can react. 5. If the reactants are mixed in the correct stoichiometric proportions, then one of the reactants will be consumed while another will be left over. 6. In finding the limiting reagent and the excess reactant, we must first have a balanced chemical equation. 7. To solve for the excess reactant, we need to subtract the total reactant from the actual reactant used. 8. The excess reactant when consumed completely, limits the reaction from continuing.
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it
Answer 1 True When that once all the limiting reactant has been com... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
6092dc1736542_210312.pdf
180 KBs PDF File
6092dc1736542_210312.docx
120 KBs Word File


