Question: Determine the Kb for a base by constructing an ICE table and using this information to construct and solve the equilibrium constant expression. Complete Parts
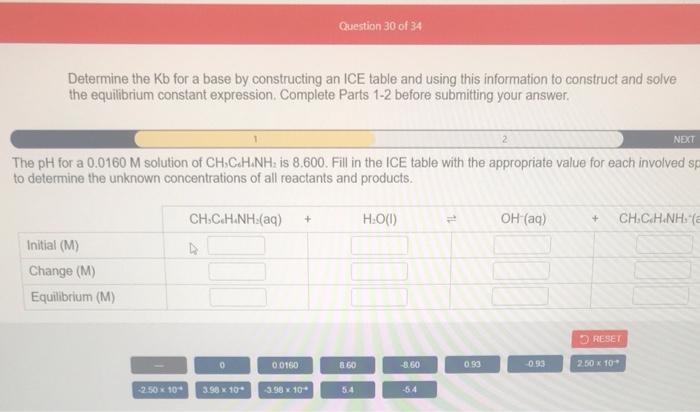
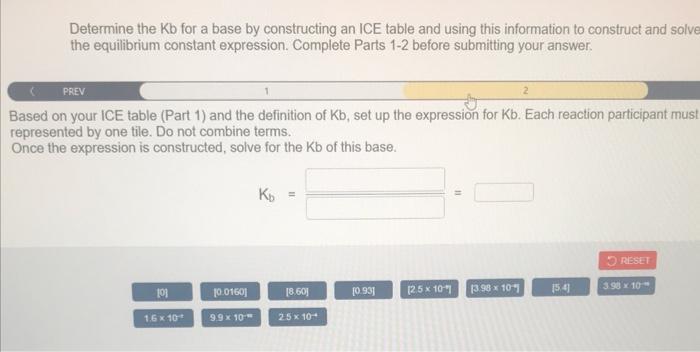
Determine the Kb for a base by constructing an ICE table and using this information to construct and solve the equilibrium constant expression. Complete Parts 12 before submitting your answer. The pH for a 0.0160M solution of CH2CHH1NH2 is 8.600. Fill in the ICE table with the appropriate value for each involved s to determine the unknown concentrations of all reactants and products. Determine the Kb for a base by constructing an ICE table and using this information to construct and solve the equilibrium constant expression. Complete Parts 1-2 before submitting your answer. PREV: ised on your ICE table (Part 1) and the definition of Kb, set up the expression for Kb. Each reaction participant mus presented by one tile. Do not combine terms. nce the expression is constructed, solve for the Kb of this base. Kb==
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


