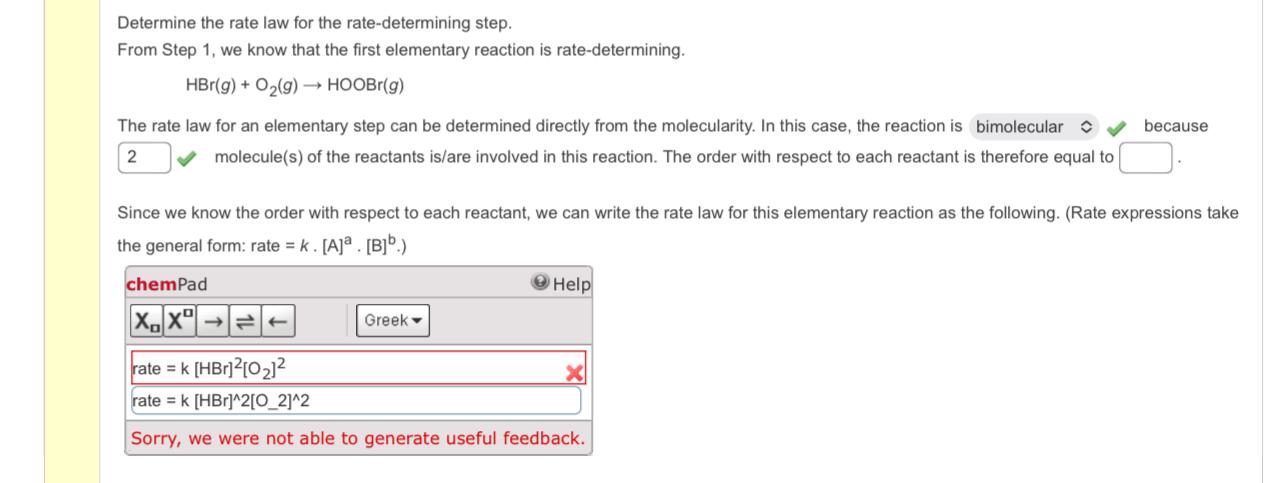
Question: Determine the rate law for the rate - determining step. From Step 1 , we know that the first elementary reaction is rate - determining.
Determine the rate law for the ratedetermining step.
From Step we know that the first elementary reaction is ratedetermining.
OBr
The rate law for an elementary step can be determined directly from the molecularity. In this case, the reaction is bimolecular hat because molecules of the reactants isare involved in this reaction. The order with respect to each reactant is therefore equal to
Since we know the order with respect to each reactant, we can write the rate law for this elementary reaction as the following. Rate expressions take the general form: rate
chemPad
Help
tablerate
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


