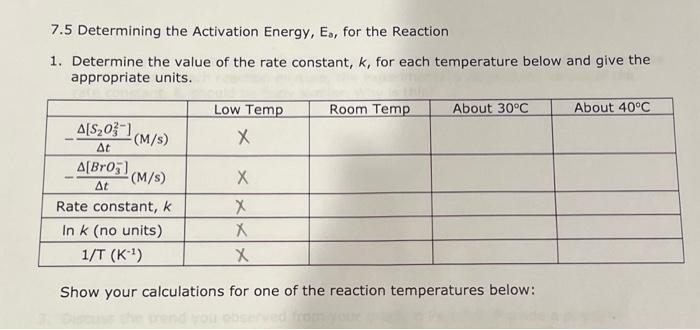
Question: Determine the value for the rate constant, k, for each temperature below and give the appropriate units. Please show all work neatly 7.5 Determining the


![\hline ReactionMixture & {[[]0(M)} & {[S2O32]0(M)} & {[BrO3]0(M)} & {[H+]0(M)} \\ \hline](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f964c7c7050_29566f964c769bfe.jpg)
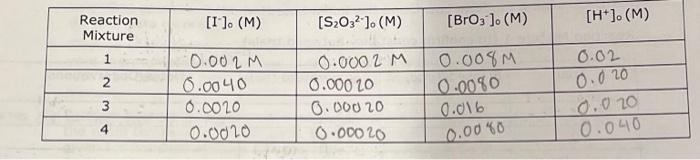
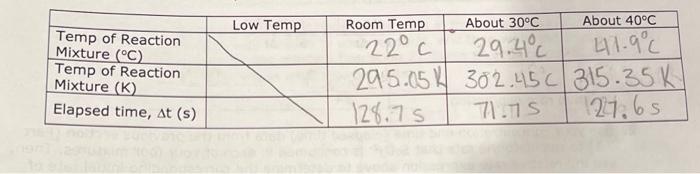
7.5 Determining the Activation Energy, Es, for the Reaction 1. Determine the value of the rate constant, k, for each temperature below and give the appropriate units. Show your calculations for one of the reaction temperatures below: \begin{tabular}{|c|c|c|c|c|} \hline ReactionMixture & {[[]0(M)} & {[S2O32]0(M)} & {[BrO3]0(M)} & {[H+]0(M)} \\ \hline 1 & 0.002M & 0.0002M & 0.008M & 0.02 \\ \hline 2 & 0.0040 & 0.00020 & 0.0080 & 0.020 \\ \hline 3 & 0.0020 & 0.00020 & 0.016 & 0.020 \\ \hline 4 & 0.0020 & 0.00020 & 0.0040 & 0.040 \\ \hline \end{tabular} \begin{tabular}{|l|c|c|c|c|} \hline & Low Temp & Room Temp & About 30C & About 40C \\ \hline TempofReactionMixture(C) & 22C & 29.4C & 41.9C \\ \hline TempofReactionMixture(K) & & 295.051 & 302.45C & 315.35K \\ \hline Elapsed time, t(s) & & 128.7S & 71.7s & 27.6S \\ \hline \end{tabular} 6I(aq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O(I)slow(clockreaction)3I2(aq)+6S2O32(aq)6I(aq)+3S4O62(aq)fast Overall reaction: BrO3(aq)+6S2O32(aq)+6H+(aq)Br(aq)+3H2O(I)+3S4O62(aq)(Equation4) Relative rates of reaction \begin{tabular}{|c|c|c|c|c|} \hline ReactionMixture & \multicolumn{1}{|c}{I]0(M)} & {[BrO3](M)} & {[H+]0(M)} & Rate (M/s) \\ \hline 1 & 0.002 & 0.004M & 0.02 & 2107 \\ \hline 2 & 0.0040 & 0.0040 & 0.02 & 4.3107 \\ \hline 3 & 0.0020 & 0.016 & 0.02 & 4.01107 \\ \hline 4 & 0.0020 & 0.0040 & 0.04 & 9107 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


