Question: Determine which reaction process is an increase in entropy. (Select ALL possible correct answers) A) 2N2H2(g) + 2NH3(g) + H2(g) B) H20(1), 0C H20(s), 0C
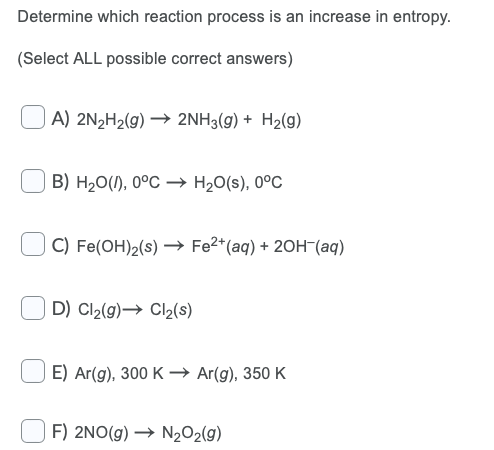
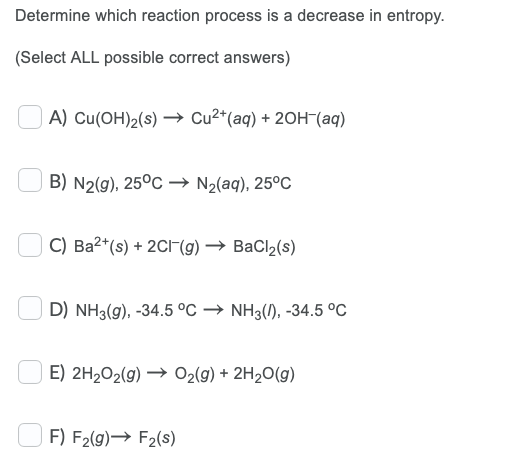
Determine which reaction process is an increase in entropy. (Select ALL possible correct answers) A) 2N2H2(g) + 2NH3(g) + H2(g) B) H20(1), 0C H20(s), 0C C) Fe(OH)2(s) Fe2+ (aq) + 2OH-(aq) + D) Cl2(g) + Cl2(S) E) Ar(g), 300 K Ar(g), 350 K F) 2NO(g) N2O2(g) Determine which reaction process is a decrease in entropy. (Select ALL possible correct answers) A) Cu(OH)2(s) Cu2+(aq) + 2OH-(aq) B) N2(g), 25C + N2(aq), 25C C) Ba2+(s) + 2C1-(g) BaCl2(s) D) NH3(g), -34.5 C NH3(1), -34.5 C E) 2H2O2(g) + O2(g) + 2H2O(9) F) F2(g) F2(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


