Question: Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below) 15-16 A gas-phase reaction A
Hi, need help with a textbook homework question from my reaction process engineering course in chemical engineering. (Picture attached below)
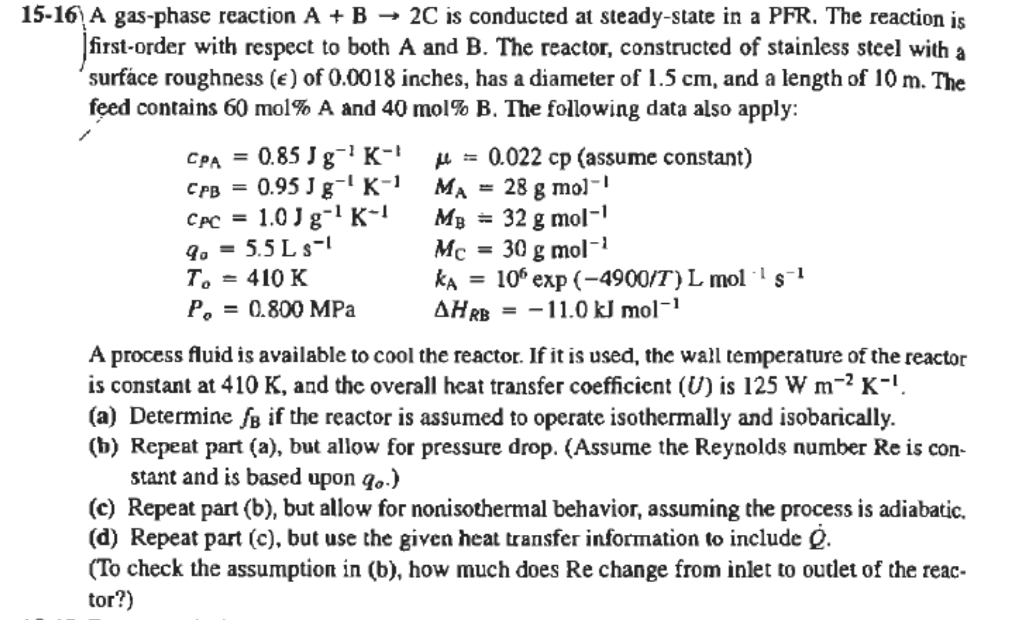
15-16 A gas-phase reaction A + B 2C is conducted at steady-state in a PFR. The reaction is first-order with respect to both A and B. The reactor, constructed of stainless steel with a 'surface roughness () of 0.0018 inches, has a diameter of 1.5 cm, and a length of 10 m. The feed contains 60 mol% A and 40 mol% B. The following data also apply: CPA 0.85 Jg'K- H = 0.022 cp (assume constant) 0.95 Jg-'K-1 MA 28 g mol-' 1.01g-K- Mg = 32 g mol-' 9. = 5.5 L 3-1 Mc = 30 g mol-1 g T. = 410 K ka = 10% exp(-4900/T) L mol 's-1 P, = 0.800 MPa = -11.0 kJ mol-1 CPB = C = AH RB A process fluid is available to cool the reactor. If it is used, the wall temperature of the reactor is constant at 410 K, and the overall heat transfer coefficient (U) is 125 W m-PK-! (a) Determine o if the reactor is assumed to operate isothermally and isobarically. (b) Repeat part (a), but allow for pressure drop. (Assume the Reynolds number Re is con- stant and is based upon qo.) (c) Repeat part (b), but allow for nonisothermal behavior, assuming the process is adiabatic. (d) Repeat part (c), but use the given heat transfer information to include e. (To check the assumption in (b), how much does Re change from inlet to outlet of the reac- tor?) 15-16 A gas-phase reaction A + B 2C is conducted at steady-state in a PFR. The reaction is first-order with respect to both A and B. The reactor, constructed of stainless steel with a 'surface roughness () of 0.0018 inches, has a diameter of 1.5 cm, and a length of 10 m. The feed contains 60 mol% A and 40 mol% B. The following data also apply: CPA 0.85 Jg'K- H = 0.022 cp (assume constant) 0.95 Jg-'K-1 MA 28 g mol-' 1.01g-K- Mg = 32 g mol-' 9. = 5.5 L 3-1 Mc = 30 g mol-1 g T. = 410 K ka = 10% exp(-4900/T) L mol 's-1 P, = 0.800 MPa = -11.0 kJ mol-1 CPB = C = AH RB A process fluid is available to cool the reactor. If it is used, the wall temperature of the reactor is constant at 410 K, and the overall heat transfer coefficient (U) is 125 W m-PK-! (a) Determine o if the reactor is assumed to operate isothermally and isobarically. (b) Repeat part (a), but allow for pressure drop. (Assume the Reynolds number Re is con- stant and is based upon qo.) (c) Repeat part (b), but allow for nonisothermal behavior, assuming the process is adiabatic. (d) Repeat part (c), but use the given heat transfer information to include e. (To check the assumption in (b), how much does Re change from inlet to outlet of the reac- tor?)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


