Question: Dibromoethane and potassium iodide react in methanol (the solvent) according to the equation: CH4Br2 + 3KI CH4 + 2KBr + Kl3 The initial rate
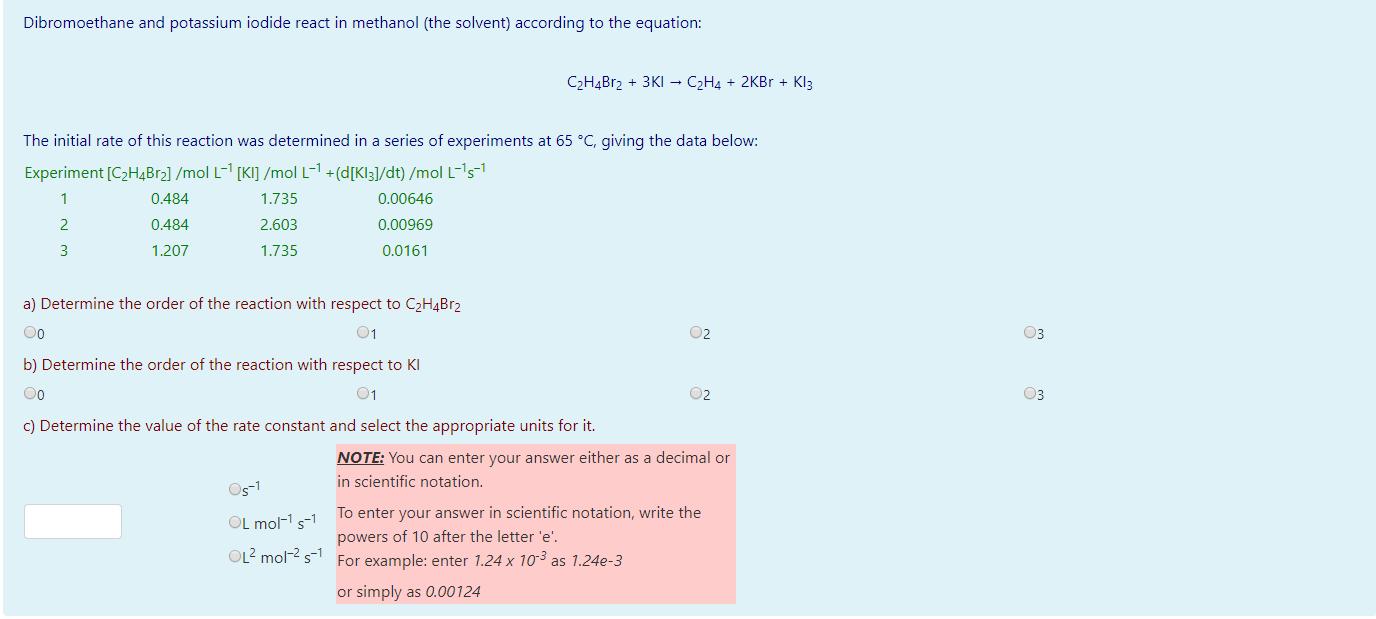
Dibromoethane and potassium iodide react in methanol (the solvent) according to the equation: CH4Br2 + 3KI CH4 + 2KBr + Kl3 The initial rate of this reaction was determined in a series of experiments at 65 C, giving the data below: Experiment [CH4Br] /mol L-1 [KI] /mol L-1 +(d[Kl3]/dt) /mol L-s- 1 0.484 1.735 0.00646 2 0.484 2.603 0.00969 3 1.207 1.735 0.0161 a) Determine the order of the reaction with respect to CH4BR2 Oo 01 02 b) Determine the order of the reaction with respect to KI Oo 01 c) Determine the value of the rate constant and select the appropriate units for it. 05-1 OL mol- s-1 OL mol- s 02 NOTE: You can enter your answer either as a decimal or in scientific notation. To enter your answer in scientific notation, write the powers of 10 after the letter 'e'. For example: enter 1.24 x 10- as 1.24e-3 or simply as 0.00124 03 03
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
a b c To find the order of reaction with respect to ... View full answer

Get step-by-step solutions from verified subject matter experts


