Question: Diffusion mechanisms and their relation to the Arrhenius temperature dependence of the diffusivity: D = D 0 exp ( - Q d R T )
Diffusion mechanisms and their relation to the Arrhenius temperature dependence of the diffusivity: exp Ideal gas constant :
a Interstitial diffusion. In class, we discussed the interstitial mechanism of diffusion in a solid, whereby an interstitial impurity atom "hops" through the lattice. The activation energy for diffusion is related to the energy barrier that the interstitial atom must overcome as it moves from one interstitial site to another. Given this, why would you expect that interstitial carbon atoms diffuse slightly more slowly than interstitial nitrogen atoms in fcc iron hint: consider the atomic radii of and The atomic radii of and are and respectively.
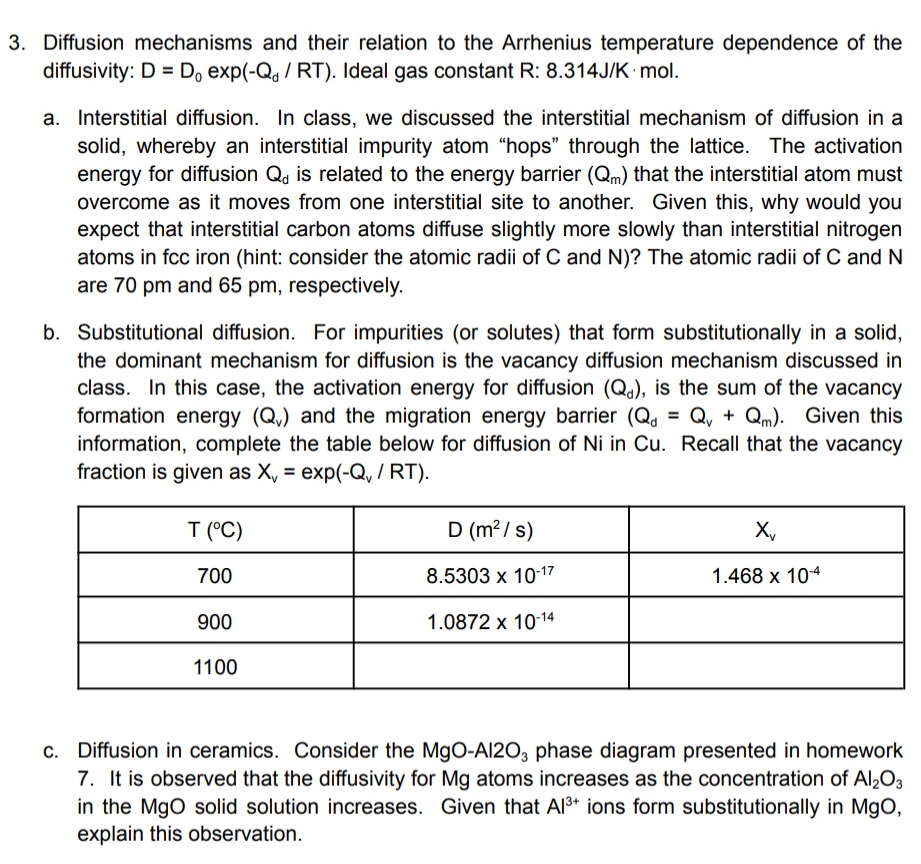
b Substitutional diffusion. For impurities or solutes that form substitutionally in a solid, the dominant mechanism for diffusion is the vacancy diffusion mechanism discussed in class. In this case, the activation energy for diffusion is the sum of the vacancy formation energy and the migration energy barrier Given this information, complete the table below for diffusion of in Recall that the vacancy fraction is given as exp
table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


