Question: Dimethyl ether production PFD, stream table, reaction kinetics, and specification of major equipment for the DME production unit are provided in the attached file. a)
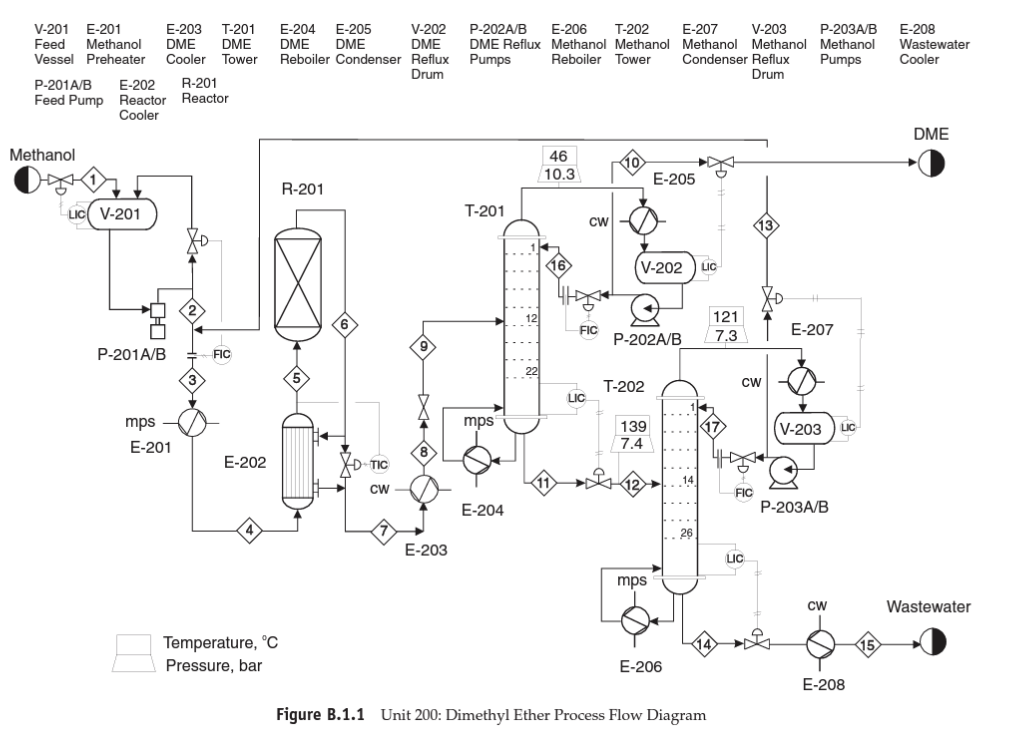
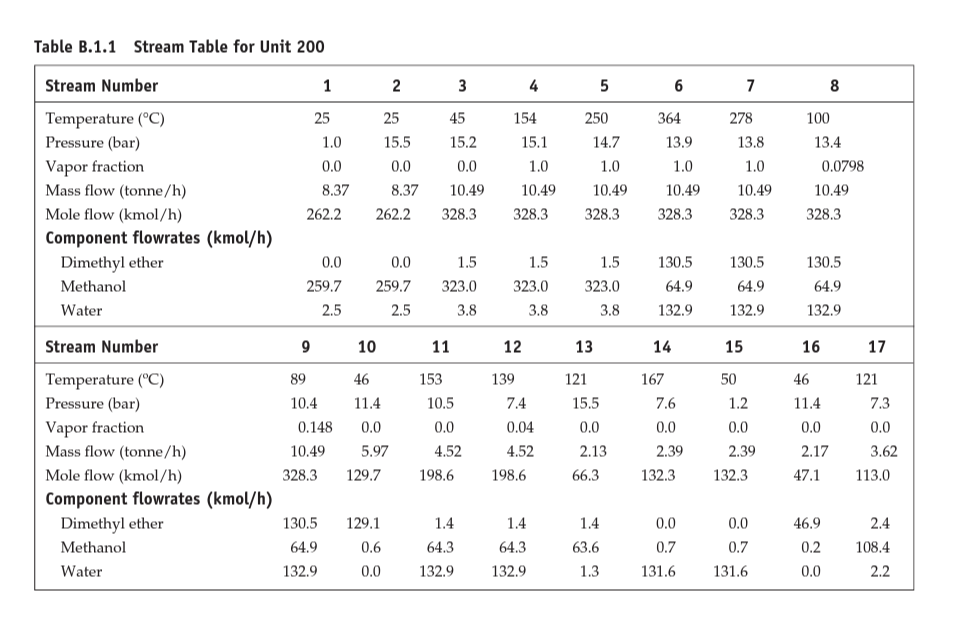

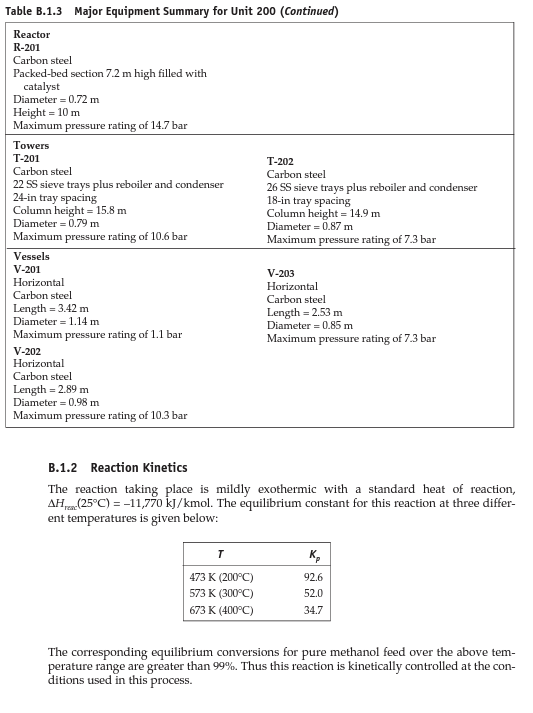

Dimethyl ether production PFD, stream table, reaction kinetics, and specification of major equipment for the DME production unit are provided in the attached file. a) Use the skills you have acquired (diagrams and conceptual chemical process design) and provide a detailed process description for the DME production process. Review Douglas method and provide detailed information about process chemistry, input/output streams structure, recycle structure(s), separation systems, utility, and the possibility of heat integration. You can use calculations from part "b" for a more detailed and specific description. (Limit: 500 words) - 15 points b) Use engineering heuristics to size the following major equipment: - E-201, E-202, E-203 (10 points), E-204 (10 extra points) - Area and type of exchanger and provide an estimation of q for E-202 - P-201 A/B, P-203 A/B (10 points) - Power, head, type of pump, efficiency - R-201 (10 points) - Length and diameter - T-201, T-202 (20 points) - Length, diameter, N and R - V-201, V-203 (10 points) - Length and diameter Follow (step-by-step) heuristic rules for each equipment and provide sufficient details and reasoning for your calculations. Compare your findings with specifications reported in Table B.1.3. Figure B.1.1 Unit 200: Dimethyl Ether Process Flow Diagram Tahla R.1.1 Straam Tahla for Ilnit onn Table B.1.2 Utility Summary Table for Unit 200 [ 1 B.1.2 Reaction Kinetics The reaction taking place is mildly exothermic with a standard heat of reaction, Hrax(25C)=11,770kJ/kmol. The equilibrium constant for this reaction at three different temperatures is given below: The corresponding equilibrium conversions for pure methanol feed over the above temperature range are greater than 99%. Thus this reaction is kinetically controlled at the conditions used in this process. The reaction takes place on an amorphous alumina catalyst treated with 10.2% silica. There are no significant side reactions at less than 400C. At greater than 250C the rate equation is given by Bondiera and Naccache [2] as: rmetlinal=k0exp[RTE0]pmethunal where k0=1.21106kmol/(m3cat.h.kPa),E0=80.48kJ/mol, and pmethemol= partial pressure of methanol ( kPa). Significant catalyst deactivation occurs at temperatures greater than 400C, and the reactor should be designed so that this temperature is not exceeded anywhere in the reactor. The design given in Figure B.1.1 uses a single packed bed of catalyst, which operates adiabatically. The temperature exotherm occurring in the reactor of 118C is probably on the high side and gives an exit temperature of 368C. However, the single-pass conversion is quite high (80%), and the low reactant concentration at the exit of the reactor tends to limit the possibility of a runaway. In practice the catalyst bed might be split into two sections, with an intercooler between the two beds. This has an overall effect of increasing the volume (and cost) of the reactor and should be investigated if catalyst damage is expected at temperatures lower than 400C. In-reactor cooling (shell-and-tube design) and cold quenching by splitting the feed and feeding at different points in the reactor could also be investigated as viable alternative reactor configurations. B.1.3 Simulation (CHEMCAD) Hints The DME-water binary system exhibits two liquid phases when the DME concentration is in the 34% to 93% range [2]. However, upon addition of 7% or more alcohol, the mixture becomes completely miscible over the complete range of DME concentration. In order to ensure that this nonideal behavior is simulated correctly, it is recommended that binary vapor-liquid equilibrium (VLE) data for the three pairs of components be used in order to regress binary interaction parameters (BIPs) for a UNIQUAC/UNIFAC thermodynamics model. If VLE data for the binary pairs are not used, then UNIFAC can be used to estimate BIPs, but these should be used only as preliminary estimates. As with all nonideal systems, there is no substitute for designing separation equipment using data regressed from actual (experimental) VLE. B.1.4 References 1. "DuPont Talks about Its DME Propellant," Aerosol Age, May and June 1982. 2. Bondiera, J., and C. Naccache, "Kinetics of Methanol Dehydration in Dealuminated H-Mordenite: Model with Acid and Basic Active Centres," Applied Catalysis 69 (1991): 139-148. ETHYLBENZENE PRODUCTION, UNIT 300 The majority of ethylbenzene (EB) processes produce EB for internal consumption within a coupled process that produces styrene monomer. The facility described here produces 80,000 tonne/y of 99.8mol% ethylbenzene that is totally consumed by an on-site styrene
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


