Question: Part II. Determination of molecular weight for ethylene glycol ATI Freezing point, C -1.9 -1.9 C 1 L Mass of solute, mass of solvent,
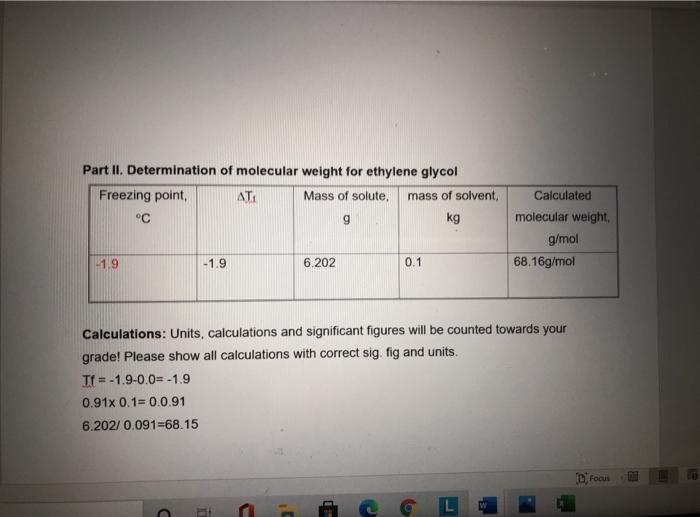
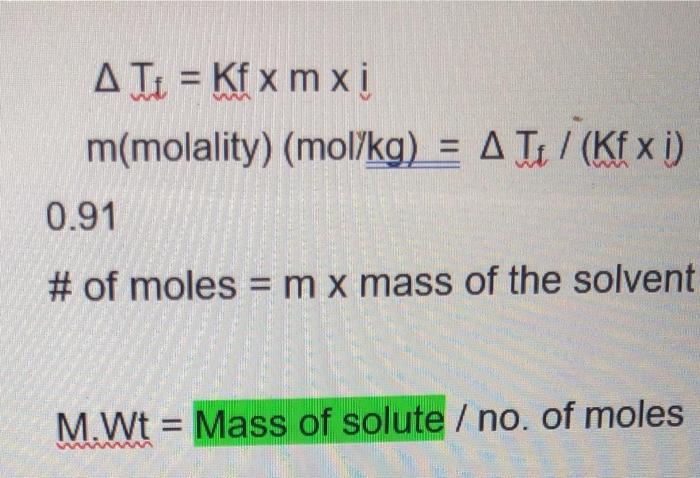
Part II. Determination of molecular weight for ethylene glycol ATI Freezing point, C -1.9 -1.9 C 1 L Mass of solute, mass of solvent, g kg C 6.202 Calculations: Units, calculations and significant figures will be counted towards your grade! Please show all calculations with correct sig. fig and units. Tf= -1.9-0.0= -1.9 0.91x 0.1=0.0.91 6.202/0.091-68.15 Cam 0.1 C Calculated W molecular weight, g/mol 68.16g/mol Focus ER T = Kf xm x i m(molality) (mol/kg) = A T / (Kfx i) 0.91 # of moles= m x mass of the solvent M.Wt Mass of solute / no. of moles
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
It seems that the images youve provided are part of an experiment to determine the molecular weight ... View full answer

Get step-by-step solutions from verified subject matter experts


