Question: Do not copy paste old solutions. Show work step by step . paste old solutions. Show work step by step 1. The molar Gibbs free
Do not copy paste old solutions. Show work step by step .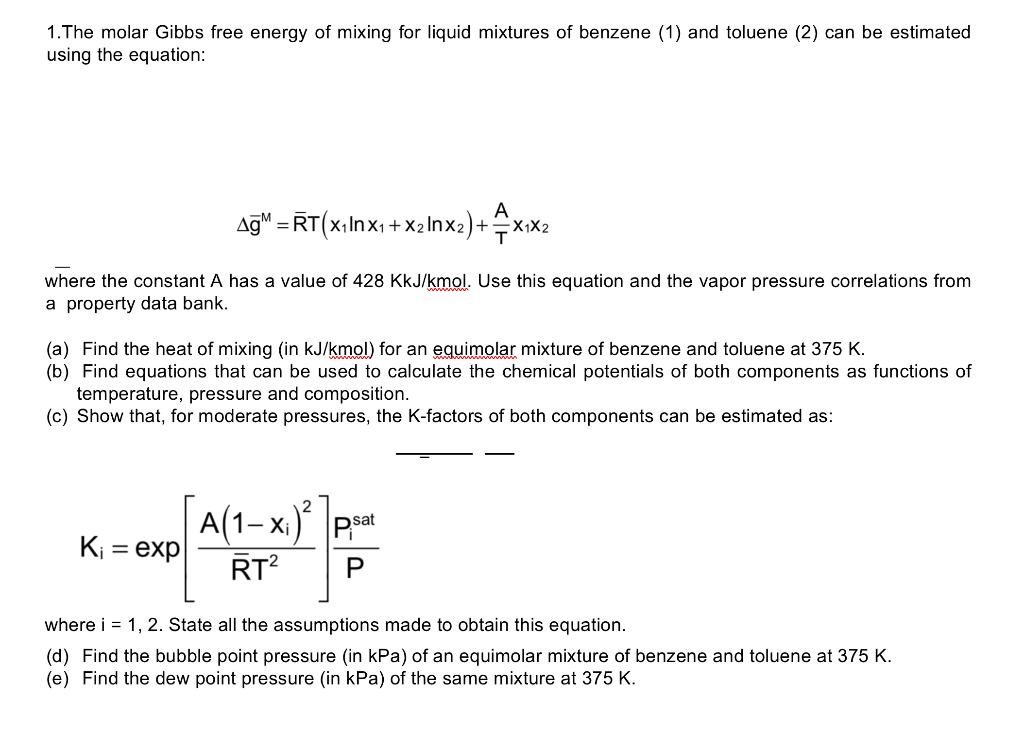 paste old solutions. Show work step by step
paste old solutions. Show work step by step
1. The molar Gibbs free energy of mixing for liquid mixtures of benzene (1) and toluene (2) can be estimated using the equation: AM = RT(x1In x1 + x2 Inx2)+ x2) + x1x2 = where the constant A has a value of 428 KkJ/kmol. Use this equation and the vapor pressure correlations from a property data bank. (a) Find the heat of mixing (in kJ/kmol) for an equimolar mixture of benzene and toluene at 375 K. (b) Find equations that can be used to calculate the chemical potentials of both components as functions of temperature, pressure and composition. (c) Show that, for moderate pressures, the K-factors of both components can be estimated as: A(1 K = exp 1- xi) psat RT? where i = 1, 2. State all the assumptions made to obtain this equation. (d) Find the bubble point pressure (in kPa) of an equimolar mixture of benzene and toluene at 375 K. (e) Find the dew point pressure (in kPa) of the same mixture at 375 K. 1. The molar Gibbs free energy of mixing for liquid mixtures of benzene (1) and toluene (2) can be estimated using the equation: AM = RT(x1In x1 + x2 Inx2)+ x2) + x1x2 = where the constant A has a value of 428 KkJ/kmol. Use this equation and the vapor pressure correlations from a property data bank. (a) Find the heat of mixing (in kJ/kmol) for an equimolar mixture of benzene and toluene at 375 K. (b) Find equations that can be used to calculate the chemical potentials of both components as functions of temperature, pressure and composition. (c) Show that, for moderate pressures, the K-factors of both components can be estimated as: A(1 K = exp 1- xi) psat RT? where i = 1, 2. State all the assumptions made to obtain this equation. (d) Find the bubble point pressure (in kPa) of an equimolar mixture of benzene and toluene at 375 K. (e) Find the dew point pressure (in kPa) of the same mixture at 375 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


